
Pinus contorta. Image by Rudi Riet, Washington, DC, United States [CC-BY-SA-2.0], via Wikimedia Commons
This study was a really good example of a home-away growth experiment, and showed some really striking results. Seeds from Pinus contorta trees of known provenance were collected in Sweden, and then grown in soil from their native Canadian sites and their introduced
Swedish sites to look for differences in aboveground and belowground biomass. Sounds simple? It would be, but they didn’t just compare fresh soils containing whatever biota could contend with the 2mm sieve. They also used sterilised soils from both countries, sterilised soils which had been cross-inoculated with soils from the opposite country, and included a fertilisation element to make it more interesting.
So what was the take-home message? The clue is in the title, but the short version is that it’s all about the soil biota. Seedlings grown in Swedish soils had much higher biomass (around 43% higher) than those grown in native their native Canadian soils, despite the Swedish soils having lower pH and nutrient availability. When seedlings were grown in sterilised soils, the soil origin had no effect on biomass. When seedlings were grown in sterilised soil from either country, but inoculated with soil biota from Sweden, biomass was again much greater than when sterilised soils were inoculated with Canadian soil biota. The effect of soil biota was even greater than the effect of fertiliser on biomass.
Considering these different trials together, it becomes clear that not only did the Swedish biota have a strong positive effect on P. contorta seedling biomass, but that the Canadian biota actually had a negative effect on biomass. This provides evidence that better growth in soils from outside of the native range is probably down to a combination of pathogen release and positive biotic associations.
However, this evidence also highlights the gap in this paper – analysis of the soil biota itself. We see the effects, but what are the actual differences in the bacterial, fungal, and/or mesofaunal communities? Are there differences in community structure? Abundances or biomass? Activity? All of these? It would be great to see data on this, especially regarding known pathogens or ectomycorrhizal fungi associates. I hope/suspect there may be another paper on the way exploring some this.
Overall, this was a really nicely designed project that asked interesting questions and addressed them in a very straightforward way. The paper itself was well written. It flows nicely and is easy to follow and understand even on a quick reading. The methods made no attempt to disguise the logistical issues associated with transporting soils half way around the world, and the data analysis and presentation was simple and direct with no unnecessary frills or risk of misinterpretation. All of these are elements that we should expect of any paper, but sometimes experiments are complex and difficult to describe, methodological detail gets glossed over in an attempt to meet word counts, and data are not easy to interpret. While it is not possible to answer all questions with a glasshouse study, or to present all data with bar graphs, it is a nice reminder of the clarity with which we should try to communicate our work.
So now that I’ve had my say, exposing my soil ecology and science communication biases, we’d love to hear what you thought about the paper. Was there anything you would have done differently? What do you think the next steps should be? What did you think of the tree provenance element of the study? It didn’t show a significant effect here, but would it be worth pursuing in other systems? How well do you think the results of this glasshouse study represent what’s happening in the natural communities?
You can let us know your take on this either in the comments or via twitter using the hashtag #psejclub. We’d also love to hear from you If you have a suggestion for a paper you’d like us to discuss, or if you want to write a post yourself!
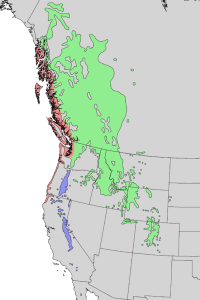
Pinus contorta native range. Image by Elbert L. Little, Jr., of the U.S. Department of Agriculture, Forest Service, USGS
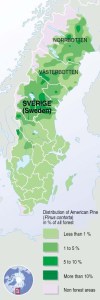
P. contorta range in Sweden. Image by Philippe Rekacewicz, UNEP/GRID-Arendal

Interesting paper; note that in the 1970s and onwards, Pinus contorta in Scotland suffered devastating attacks from our native pine beauty moth, Panolis flammea.
Hi Sarah and the PSEJClub,
I found this study to be well-designed with a clear presentation of the findings and the implications of those findings; it was a really clearly laid out study which I appreciate. I look forward to a future paper that characterizes the Swedish and Canadian soil communities, and I suspect the authors may be working on that next- it seems like the logical next step. I am curious to know if there are specific soil organisms responsible for the enhanced growth (if species ID or a specific taxa is the key player here) and the soils have overall fairly simimlar communities, or if the soil communities are radically different (or any combination along that gradient). This study was great for assessing home and away effects of soils on tree growth, and I’m looking forward to seeing additional work on specifying the soil biotic community to shed some light on the mechanism behind the observed growth patterns.
As with introduced species, I find my mind quickly wanders into global change territory. I am curious about how some introduced species and biogeographic differences in soil communities may interact over time given changing climatic regimes, the addition and loss of co-occurring species, etc. I wonder if the relationships observed in this study would persist, possibly be enhanced or conversely diminished given future climate projections for both Canada and Sweden.
Thanks for the comments. Simon, that’s really interesting. Maybe what happens aboveground in terms of herbivory, pathogens, etc could outweigh or at least moderate the positive impacts of the belowground biota. From the range map, it looks like P contorta is doing pretty well in Sweden, but perhaps periodic attacks from things like the moth you mentioned keep it in check.
Relena, my mind jumps to questions about global change too. As the authors mentioned in the discussion, ectomycorrhizal fungi effects on plants can swap from positive to parasitic depending on context, so changes in climate, nutrient deposition, and other abiotic factors may alter the biotic associations, both home and away, even before we begin thinking about invasions, range shifts, etc that also are influenced by global change.
Again, I think it comes back to what’s actually in the soil, and look forward to finding out if that data is published. There’s also the question of how stable the soil biotic community is, spatially and temporally, Do you see the same taxa (and relationships) in a dry year as in a wet year? Are the same taxa driving the interactions across the range?
I’d also be interested to know more about productivity in forest plots in both regions, and how it compares to what is seen in the glasshouse. If there are differences, what factors are most important in driving those differences? I imagine variability in temperature and precipitation would play an important role, as well as (aboveground) herbivores and pathogens and possibly variations in the soil biota. Perhaps someone has already done some of this work?
While I think this paper is interesting from the perspective of how belowground communities might influence range shifts, I don’t think the statistical approach employed is appropriate given the soil and seed collection design. Specifically, the authors treat soil origin (Canada or Sweden) as a fixed factor, but there are multiple provenances where soils were sampled and thus these subsites should be nested within the larger regions and treated as a random effect in the model. A similar approach should be used for the seedlings, and the by not accounting for this variation I believe the authors have inflated their probability of detecting significant effects. Perhaps a clearer explanation of the the stats approach would help shed light on these issues, but it does not appear that any appreciation of these important components of variation in the models was used. These things are extremely important to consider when different PSF experimental designs and analyses have been shown to lead to varying results given identical starting materials (see Brinkman et al. 2010).
Interesting points about the statistical approach. I thought that site should have been included as a random effect too, but I’m no stats whiz (yet) and was talked around during our journal club discussions. Given the similarity of the soils, the fact the country-specific soil biota inocula were from a mix of all soils from the country rather than a specific site, and the magnitude of the results they reported, I would be surprised if the random effect would have made a huge difference. That said, it would have been good to see it tested and reported, or a solid justification for why they didn’t.