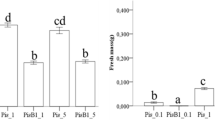
Abstract
Putative endophytes of Miscanthus × giganteus were isolated, and screened in the laboratory, greenhouse and field for their plant growth promoting properties in this host. Pantoea ananatis and Pseudomonas savastanoi were the predominant bacteria in leaves whereas other pseudomonads prevailed in roots. Almost all fungal endophytes belonged to the Pezizomycotina and most were isolated from roots; Fusarium oxysporum was most abundant, followed by the genera Periconia, Exophiala, Microdochium and Leptodontidium. All endophytic groups produced phytohormones and some bacteria also produced siderophores, solubilised P and exhibited ACC-deaminase activity in vitro. In subsequent pot experiments with pre-selected endophytes, several isolates including pseudomonads, Variovorax paradoxus, Verticillium leptobactrum, Halenospora sp. and Exophiala sp. enhanced Miscanthus growth in gamma-sterilised soil. These promising Miscanthus-derived isolates were tested either as single or mixed inocula along with a mixed bacterial inoculum originating from poplar. No significant effects of inocula were detected in a pot experiment in non-sterilised soil. On two marginal field sites the mixture of bacterial endophytes from poplar had a consistently negative effect on survival and growth of Miscanthus. Contrarily, mixtures consisting of bacteria or fungi originating from Miscanthus promoted growth of their host, especially on the heavy metals-polluted site. The combination of bacteria and fungi was inferior to the mixtures consisting of bacteria or fungi alone. Our observations indicate extensive potential of mixed bacterial and fungal endophytic inocula to promote establishment and yield of Miscanthus grown on marginal and polluted land and emphasise the necessity to test particular microbial-plant host combinations.
Graphical Abstract
Morphotypes of fungi isolates from Miscanthus × giganteus








Similar content being viewed by others
References
Alexander DB, Zuberer DA (1991) Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol Fertil Soils 12:39–45
Babu AG, Shea PJ, Sudhakar D, Jung I-B, Oh B-T (2015) Potential use of Pseudomonas koreensis AGB-1 in association with Miscanthus sinensis to remediate heavy metal(loid)-contaminated mining site soil. J Environ Manage 151:160–166. https://doi.org/10.1016/j.jenvman.2014.12.045
Bakker PAHM, Pieterse CMJ, van Loon LC (2007) Induced systemic resistance by fluorescent Pseudomonas sp. Phytopathology 97:239–243. https://doi.org/10.1094/PHYTO-97-2-0239
Bashan Y, Kamnev AA, de-Bashan LE (2013) Tricalcium phosphate is inappropriate as a universal selection factor for isolating and testing phosphate-solubilizing bacteria that enhance plant growth: a proposal for an alternative procedure. Biol Fertil Soils 49(4):65–479. https://doi.org/10.1007/s00374-012-0737-7
Batzer JC, Weber RWS, Mayfield DA, Gleason ML (2016) Diversity of the sooty blotch and flyspeck complex on apple in Germany. Mycol Prog 15:2. https://doi.org/10.1007/s11557-015-1145-9
Berbee ML (2001) The phylogeny of plant and animal pathogens in the Ascomycota. Physiol Mol Plant P 59:165–187
Bills G, Platas G, Pelaez F, Masurekar P (1999) Reclassification of a pneumocandin-producing anamorph, Glarea lozoyensis gen. et sp. nov., previously identifed as Zalerion arboricola. Mycol Res 103:179–192
Burd GI, Dixon DG, Glick BR (2000) Plant growth-promoting bacteria that decrease heavy metal toxicity in plants. Can J Microbiol 46:237–245
Cherkaoui A, Hibbs J, Emonet S, Tangomo M, Girard M, Francois P, Jacques Schrenzel J (2016) Comparison of two matrix-assisted laser desorption ionization–time of flight mass spectrometry methods with conventional phenotypic identification for routine identification of bacteria to the species level. J Clinic Microbiol 48:1169–1175
Cole J, Chai B, Farris RJ, Wang Q, Kulam SA, McGarrell MD, Garrity GM, Tiedje JM (2005) The Ribosomal Database Project (RDP-II): sequences and tools for high-throughput rRNA analysis. Nucleic Acids Res 33:294–296
Cope-Selby N, Cookson A, Squance M, Donnison I, Flavell R, Farrar K (2017) Endophytic bacteria in Miscanthus seed: implications for germination, vertical inheritance of endophytes, plant evolution and breeding. GCB Bioenergy 9:57–77. https://doi.org/10.1111/gcbb.12364
Covarelli L, Beccari G, Tosi L (2012) Miscanthus rhizome rot: a potential threat for the establishment and the development of biomass cultivations. Biomass Bioenergy 46:263–269. https://doi.org/10.1016/j.biombioe.2012.08.018
Davis MP, David MB, Voigt TB, Mitchell CA (2015) Effect of nitrogen addition on Miscanthus × giganteus yield, nitrogen losses, and soil organic matter across five sites. GCB Bioenergy 7:1222–1231. https://doi.org/10.1111/gcbb.12217
de Freitas JR, Germida JJ (1991) Pseudomonas cepacia and Pseudomonas putida as winter wheat inoculants for biocontrol of Rhizoctonia solani. Can J Microbiol 37:780–784
de Abreu LM, Almeida AR, Salgado M, Pfenning LH (2010) Fungal endophytes associated with the mistletoe Phoradendron perrottettii and its host tree Tapirira guianensis. Fungal Progress 4:559–566. https://doi.org/10.1007/s11557-010-0663-8
Dimkpa CO, Merten D, Svatoš A, Büchel G, Kothe E (2009) Metal-induced oxidative stress impacting plant growth in contaminated soil is alleviated by microbial siderophores. Soil Biol Biochem 41:154–162
Duffy BK, Defago G (1997) Zinc improves biocontrol of Fusarium crown and root rot of tomato by Pseudomonas fluorescens and represses the production of pathogen metabolites inhibitory to bacterial antibiotic biosynthesis. Phytopathology 87:1250–1257
Eskes AB, Mendes MDL, Robbs CF (1991) Laboratory and field studies on parasitism of Hemileia vastatrix with Verticillium lecani and V. leptobactrum. Café Cacao Thé 35:275–282
Etesami H, Alikhani HA, Hosseini HM (2015) Indole-3-acetic acid (IAA) production trait, a useful screening to select endophytic and rhizosphere competent bacteria for rice growth promoting agents. MethodsX 2:72–78. https://doi.org/10.1016/j.mex.2015.02.008
FAO (2006) World reference base for soil resources. A framework for international classification, correlation and communication. Food and Agriculture Organisation of the United Nations, Rome
Farrar K, Bryant D, Cope-Selby N (2014) Understanding and engineering beneficial plant –microbe interactions: plant growth promotion in energy crops. Plant Biotechnol J 12:1193–1206. https://doi.org/10.1111/pbi.12279
Garrido-Sanz D, Meier-Kolthoff JP, Göker M, Martín M, Rivilla R, Redondo-Nieto M (2016) Genomic and genetic diversity within the Pseudomonas fluorescens complex. PLoS ONE 11(2):e0150183. https://doi.org/10.1371/journal.pone.0150183
Hajšlova J, Fenclova M, Zachariašova M (2013) Methodology for the rapid screening of isolates of endophytic microorganisms and identification of strains with phytohormonal activity [in Czech]. ISBN 978-80-7080-869-6
Heaton EA, Dohleman FG, Long SP (2008) Meeting US biofuel goals with less land: the potential of Miscanthus. Glob Change Biol 14:2000–2014. https://doi.org/10.1111/j.1365-2486.2008.01662.x
Hoffman MT, Gunatilaka MK, Wijeratne K, Gunatilaka L, Arnold AE (2013) Endohyphal bacterium enhances production of indole-3-acetic acid by a foliar fungal endophyte. PLoS ONE 8:e73132. doi.https://doi.org/10.1371/journal.pone.0073132
Indrasumunar A, Dart PJ, Menzies NW (2011) Symbiotic effectiveness of Bradyrhizobium japonicum in acid soils can be predicted from their sensitivity to acid soil stress factors in acidic agar media. Soil Biol Biochem 43:2046–2052. https://doi.org/10.1016/j.soilbio.2011.05.022
ISO 10390 (2005) Soil quality: determination of pH. International Organization for Standardization, ISO
Jiang F, Chen L, Belimov AA, Shaposhnikov AI, Gong F, Meng X, Hartung W, Jeschke DW, Davies WJ, Dodd IC (2012) Multiple impacts of the plant growth-promoting rhizobacterium Variovorax paradoxus 5C-2 on nutrient and ABA relations of Pisum sativum. J Exp Bot 63:6421–6430. https://doi.org/10.1093/jxb/ers301
Kempf H-J, Wolf G (1989) Erwinia herbicola as a biocontrol agent of Fusarium culmorum and Puccinia recondita f. sp. tritici on Wheat. Phytopathology 79:990–994
Khan Z, Doty SL (2009) Characterisation of bacterial endophytes in sweet potato plants. Plant Soil 322:197–207. https://doi.org/10.1007/s11104-009-9908-1
Khan AL, Hamayun M, Waqas M, Kang S-M, Kim Y-H, Kim D-H, Lee I-J (2012a) Exophiala sp. LHL08 association gives heat stress tolerance by avoiding oxidative damage to cucumber plants. Biol Fertil Soils 48:519–529. https://doi.org/10.1007/s00374-011-0649-y
Khan AL, Hamayun M, Kang S-M, Kim Y-H, Jung H-Y, Lee J-H, Lee I-J (2012b) Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress: an example of Paecilomyces formosus LHL10. BMC Microbiol 12:32. https://doi.org/10.1186/1471-2180-12-3
Kim HJ, Lee JH, Kang BR, Rong X, Gardener BBM, Ji HJ, Park CS, Kim YC (2012) Draft genome sequence of Pantoea ananatis B1-9, a nonpathogenic plant growth-promoting bacterium. J Bacteriol 194:729. https://doi.org/10.1128/JB.06484-11
Knoth JL, Kim S-H, Tell GJ, Dothy SL (2013) Effects of cross host species inoculation of nitrogen fixing endophytes on growth and leaf physiology of maize. GCB Bioenergy 5:408–418. https://doi.org/10.1111/gcbb.12006
Koubek J, Uhlík O, Jecná K, Junková P, Vrkoslavová J, Lipov J, Kurzawova V, Macek T, Macková M (2012) Whole-cell MALDI-TOF: rapid screening method in environmental microbiology. Int Biodeter Biodegr 69:82–86. https://doi.org/10.1016/j.ibiod.2011.12.007
Kuffner M, De Maria S, Puschenreiter M, Fallmann K, Wieshammer G, Gorfer M, Strauss J, Rivelli AR, Sessitsch A (2010) Culturable bacteria from Zn- and Cd-accumulating Salix caprea with differential effects on plant growth and heavy metal availability. J Appl Microbiol 108:1471–1484. https://doi.org/10.1111/j.1365-2672.2010.04670.x
Lane DJ (1991) 16S/23S rRNA sequencing. Nucleic acid techniques. In: Stackebrandt E, Goodfellow M (eds) Bacterial systematics. Wiley, New York, pp 115–175
Li J, Kremer RJ (2006) Growth response of weed and crop seedlings to deleterious rhizobacteria. Biol Control 39:58–65
Li Z, Chang S, Lin L, Li Y, An Q (2011a) A colorimetric assay of 1-aminocyclopropane-1-carboxylate (ACC) based on ninhydrin reaction for rapid screening of bacteria containing ACC deaminase. Lett Appl Microbiol 53:178–185. https://doi.org/10.1111/j.1472-765X.2011.03088.x/full
Li L, Liu MJ, Zhang XT, Zhang HB, Sha T, Zhao ZW (2011b) Improved tolerance of maize (Zea mays L.) to heavy metals by colonization of a dark septate endophyte (DSE) Exophiala pisciphila. Sci Total Environ 409:1069–1074. https://doi.org/10.1016/j.scitotenv.2010.12.012
Li D, Voigt TB, Kent AD (2016) Plant and soil effects on bacterial communities associated with Miscanthus × giganteus rhizosphere and rhizomes. GCB Bioenergy 8:183–193. https://doi.org/10.1111/gcbb.12252
Likar M, Regvar M (2013) Isolates of dark septate endophytes reduce metal uptake and improve physiology of Salix caprea L. Plant Soil 370:593–604. https://doi.org/10.1007/s11104-013-1656-6
Linde-Laursen I (1993) Cytogenetic analysis of Miscanthus ‘Giganteus’, an interspecific hybrid. Hereditas 119:297–300
Ma Y, Prasad MNV, Rajkumar M, Freitas H (2011) Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol Adv 29:248–258. https://doi.org/10.1016/j.biotechadv.2010.12.001
Mandyam KG, Jumpponen A (2015) Mutualism-parasitism paradigm synthesized from results of root endophyte models. Front Microbiol 5:776. https://doi.org/10.3389/fmicb.2014.00776
Mejri D, Gamalero E, Tombolini R, Musso C, Massa N, Berta G, Souissi T (2010) Biological control of great brome (Bromus diandrus) in durum wheat (Triticum durum): specificity, physiological traits and impact on plant growth and root architecture of the fluorescent pseudomonad strain X33d. Biocontrol 55:561–572. https://doi.org/10.1007/s10526-010-9285-y
Moll J, Hoppe B, Konig S, Wubet T, Buscot F, Kruger D (2016) Spatial distribution of fungal communities in an arable soil. PLoS ONE 11:e0148130. doi.https://doi.org/10.1371/journal.pone.0148130
Moore PD, Chapman SB (1986) Methods in plant ecology, 2nd edn. Blackwell, Oxford
Nair A, Juwarkar AA, Singh SK (2007) Production and characterization of siderophores and its application in arsenic removal from contaminated soil. Water Air Soil Poll 180:199–212. https://doi.org/10.1007/s11270-006-9263-2
Nirenberg HI, O’Donnell K (1998) New Fusarium species and combinations within the Gibberella fujikuroi species complex. Mycologia 90:434–458
Nsanganwimana F, Pourrot B, Mensch M, Douay F (2014) Suitability of Miscanthus species for managing inorganic and organic contaminated land and restoring ecosystem services: a review. J Environ Manage 143:123–124. https://doi.org/10.1016/j.jenvman.2014.04.027
Olsen RS, Sommers LE (1982) Phosphorus. In: Page AL et al (eds) Methods in soil analysis, Part 2, chemical and microbiological properties, agronomy monograph 9.2. Agronomy series 9, ASAS publications. American Society of Agronomy, Soil Science Society of America. Madison, pp 403–430
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant 118:10–15
Pereira SIA, Castro PML (2014) Diversity and characterization of culturable bacterial endophytes from Zea mays and their potential as plant growth-promoting agents in metal-degraded soils. Environ Sci Pollut R 21:14110–14123. https://doi.org/10.1007/s11356-014-3309-6
Pii Y, Mimmo T, Tomasi N, Terzano R, Cesco S, Crecchio C (2015) Microbial interactions in the rhizosphere: beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol Fertil Soils 51:403–415. https://doi.org/10.1007/s00374-015-0996-1
Rajkumar M, Ae N, Freitas H (2009) Endophytic bacteria and their potential to enhance heavy metal phytoextraction. Chemosphere 77:153–160. https://doi.org/10.1016/j.chemosphere.2009.06.047
Reddy CA, Saravanan RS (2013) Polymicrobial multi-functional approach for enhancement of crop productivity. In: Gadd GM, Sariaslani S (eds) Advances in applied microbiology. Oxford Academic, Oxford, pp 53–113
Regaieg H, Ciancio A, Raouani NH, Rosso L (2011) Detection and biocontrol potential of Verticillium leptobactrum parasitizing Meloidogyne spp. World J Microb Biot 27:1615–1623. https://doi.org/10.1007/s11274-010-0615-0
Schmidt CS, Lovecká P, Mrnka L, Vychodilová A, Strejček M, Fenclová M, Demnerová K (2017) Distinct communities of poplar endophytes on an unpolluted and a risk elements-polluted site and their plant growth promoting potential in vitro. Microb Ecol, https://doi.org/10.1007/s00248-017-1103-y
Schulz B, Boyle C (2005) The endophytic continuum. Mycol Res 109:661–686
Sharp RG, Chen L, Davis WJ (2011) Inoculation of growing media with the rhizobacterium Variovorax paradoxus 5C-2 reduces unwanted stress responses in hardy ornamental species. Sci Hortic 129:804–811
Shrestha P, Szaro TM, Bruns TD, Taylor JW (2011) Systematic search for cultivatable fungi that best deconstruct cell walls of Miscanthus and sugarcane in the field. Appl Environ Microb 77:5490–5504. https://doi.org/10.1128/AEM.02996-10
Stenstrom E, Ndobe NE, Jonsson M, Stenlid J, Menkis A (2013) Root-associated fungi of healthy-looking Pinus sylvestris and Picea abies seedlings in Swedish forest nurseries. Scand J Forest Res 29:12–21
Sun X, Ding Q, Hyde KD, Guo LD (2012) Community structure and preference of endophytic fungi of three woody plants in a mixed forest. Fungal Ecol 5:624–632
Sundara-Rao WVB, Sinha MK (1963) Phosphate dissolving microorganisms in the soil and rhizosphere. Indian J Agric Sci 33:272–278
Tóth B, Csösz M, Dijksterhuis J, Frisvad JC, Varga J (2007) Pithomyces chartarum as pathogen on wheat. J Plant Pathol 89:405–408
Unterseher M, Schnittler M (2009) Dilution-to-extinction cultivation of leaf-inhabiting endophytic fungi in beech (Fagus sylvatica L.)—different cultivation techniques influence fungal biodiversity assessment. Mycol Res 113:645–654. https://doi.org/10.1016/j.mycres.2009.02.002
Villaño D, Fernández-Pachón MS, Moyá ML, Troncoso AM, García-Parrilla MC (2007) Radical scavenging ability of polyphenolic compounds towards DPPH free radical 1. Talanta 71:230–235. https://doi.org/10.1016/j.talanta.2006.03.050
Wald J, Hroudová M, Jansa J, Vrchotová B, Macek T, Uhlík O (2015) Pseudomonads rule degradation of polyaromatic hydrocarbons in aerated sediment. Front Microbiol 6:1268. doi.https://doi.org/10.3389/fmicb.2015.01268
Wanat N, Austruy A, Joussein E, Soubrand M, Hitmi A, Gauthier-Moussard C, Lenain J-F, Vernay P, Munch JC, Pichon M (2013) Potentials of Miscanthus × giganteus grown on highly contaminated technosols. J Geochem Explor 126–127:78–84. https://doi.org/10.1016/j.gexplo.2013.01.001
Weller DM (2007) Pseudomonas biocontrol agents of soilborne pathogens: looking back over 30 years. Phytopathology 97:250–256. https://doi.org/10.1094/PHYTO-97-2-0250
Weyens N, van der Lelie D, Taghavi S, Newman L, Vangronsveld J (2009) Exploiting plant–microbe partnerships to improve biomass production and remediation. Trends Biotechnol 27:591–598. https://doi.org/10.1016/j.tibtech.2009.07.006
White TJ, Bruns TD, Lee S, Taylor J (1990) Analysis of phylogenetic relationship by amplification and direct sequencing of ribosomal RNA genes. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press Inc., New York, pp 315–322
Zadok JC, Chang TT, Konzak A (1974) A decimal code for the growth stages of cereals. Weed Res 14:415–421
Zdor RE, Alexander CM, Kremer RJ (2007) Weed suppression by soil bacteria is affected by formulation and soil properties. Commun Soil Sci Plan 36:1289–1299. https://doi.org/10.1081/CSS-200056933
Zinniel DK, Lambrecht P, Harris NB, Feng Z, Kuczmarski D, Higley P, Ishimaru CA, Arunakumari A, Barletta RG, Vidaver AK (2002) Isolation and characterization of endophytic colonizing bacteria from agronomic crops and prairie plants. Appl Environ Microbiol 68:2198–2208
Acknowledgements
We thank MSc. Dušan Kunc and RNDr. Helena Koblihová for skilful technical assistance.
Funding
This research was funded by the Technological Agency of the Czech Republic, Contract No. TA03011184, and by the Czech Academy of Sciences (long-term research development project RVO 67985939).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
No animals or data from human participants were involved in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schmidt, C.S., Mrnka, L., Frantík, T. et al. Plant growth promotion of Miscanthus × giganteus by endophytic bacteria and fungi on non-polluted and polluted soils. World J Microbiol Biotechnol 34, 48 (2018). https://doi.org/10.1007/s11274-018-2426-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-018-2426-7