Evolutionary and Ecological Explanations for the Elevational Flexibility of Several East African Bird Species Complexes
- 1Natural History Museum of Denmark, Center for Macroecology, Evolution and Climate, GLOBE Institute, University of Copenhagen, Copenhagen, Denmark
- 2Museum of Vertebrate Zoology, Department of Integrative Biology, University of California, Berkeley, Berkeley, CA, United States
- 3Fitzpatrick Institute of African Ornithology, DST-NRF Centre of Excellence, Department of Biological Sciences, University of Cape Town, Cape Town, South Africa
Africa’s montane areas are broken up into several large and small units, each isolated as forest-capped “sky islands” in a “sea” of dry lowland savanna. Many elements of their biota, including montane forest birds, are shared across several disjunct mountains, yet it has been difficult to rigorously define an Afromontane forest avifauna, or determine its evolutionary relationships with the birds of the surrounding lowland forests. In order to trace the historical relationship between lowland and highland avifaunas, we review cases of species or groups of closely related species with breeding populations at different elevations, and use phylogeographic methods to explore the historical connections between such populations within the biodiversity hotspot of East Africa. The study reveals several idiosyncratic patterns, but also a prominent number of cases of gene flow between populations in southern areas, mainly around the Malawi Rift, and mountains and coastal forests to the north, close to the equator. This may reflect more continuous past distributions through northern Mozambique and coastal Tanzania, or seasonal migrations between areas with different rainfall regimes. Over time, these distributional dynamics have resulted in a higher persistence of lineages, and an accumulation of forest-dependent lineages within the Eastern Arc Mountains of Tanzania and the northern part of the coastal forest mosaic.
Introduction
The Afromontane region comprises punctuated chains of mountains, which mostly follow the East African rift systems, and are characterized by a distinct botanical assemblage in areas above 1,500–2,000 m in elevation (White, 1981). This mountainous region comprises volcanoes as well as uplifted enclaves of ancient crystalline bedrock extending from: (1) the Ethiopian Highlands, through (2) the Kenyan Highlands and northern Tanzania, (3) East Congo/Albertine Rift, (4) the Eastern Arc Mountains, which run from southeastern Kenya diagonally across Tanzania, to (5) the highlands of Malawi, continuing through the Chimanimani Mountains of Zimbabwe/Mozambique to South Africa, with the (6) Cameroon Highlands and (7) Angolan Highlands as isolated montane areas near the west coast of the continent. Each of these montane areas are themselves fragmented into small and large mountain blocks isolated in a “sea” of lowland savanna, and these montane areas are often referred to as “islands in the sky,” an archipelago of montane habitat within the larger expanse of the African continent. The archipelago-like distributions of mountains within and among African montane areas of endemism, differ fundamentally from other large and biologically diverse montane systems such as the Himalayas, Andes, or Rocky Mountains, where elevational bands of uniform vegetation have much greater linear continuity along the mountain range.
In eastern Africa, approximately half of all species of forest birds are confined to evergreen montane forests, which are generally recognized as a distinct ecological zone from the semi-deciduous forests of the coastal zone, known as the “Zanzibar-Inhambane coastal forest mosaic.” In spite of this, attempts to divide Africa into biochoria with distinct biota, have failed to identify a distinct area unit for Afromontane birds (Diamond and Hamilton, 1980; Crowe and Crowe, 1982; deKlerk et al., 2002; Linder et al., 2012; Holt et al., 2013). This is primarily because the small and patchy distribution of many Afromontane species and the high turnover across sites provide little connectivity in cluster analyses. Further, the rather coarse geographical grid (often 1° squares) that is typically used in such analyses, includes a greater number of species from the non-montane habitat matrix, which creates greater statistical connectivity with the surrounding landscapes (deKlerk et al., 2002; Linder et al., 2012). While only some 15% of the forest-associated birds of eastern Africa are endemic to the coastal forests, most other non-montane forest birds are quite widespread, occurring wherever there are patches of semi-evergreen vegetation on floodplains and in the foothills of montane highlands. Finally, some African bird species are patchily distributed both in highland and lowland habitats (e.g., East Coast Akalat Sheppardia gunningi, Fjeldså et al., 2000), and several birds of montane forests are phylogenetically nested within clades of lowland birds (and vice versa), suggesting dynamic shifts between lowland- and highland-breeding. The boundary between the lowland and highland avifaunas appears therefore to be fuzzy, and contributes to making biogeographic subdivisions for African birds challenging (Dowsett, 1986; Bowie, 2003).
To date, most phylogeographic studies of Afromontane birds have focused on discrete groups of taxa that diversified across the described mountain regions. In this article, we focus instead on species with mixed elevational distributions to explore the variation in distribution patterns and search for historical links between populations breeding in cool highland forests and semi-evergreen habitats in the hot lowlands. We also try to determine whether flexibility in elevational distribution is associated with specific ecologies. We restrict our study to the Tanzania-Malawi Rift Mountains and the adjacent coastal forest mosaic, which together constitute the “Eastern Afromontane Biodiversity Hotspot” (Mittermeier et al., 2004), where high species diversity has accumulated in mountains where forests persisted – likely on a permanent basis – since before the break-up of the Pan-African rainforest in the Miocene, when large parts of Africa changed to become dominated by savanna and mixed-woodlands (Lovett and Wasser, 1993).
We describe cases of bird species, or groups of closely related species, which comprise distinctive populations of breeding individuals that occupy both highland and lowland forest habitats, as well as montane species, whose present distributions seem to indicate past historical connections across lowland areas. Based on the diversity of observed distribution patterns of birds in the Eastern Afromontane Biodiversity Hotspot, we aim to address the following questions: (1) Are disjunct elevational distributions of African bird species a rare anomaly, or can we find recurring patterns, and if so, (2) how can we explain the shifts in ecology that must have taken place? (3) Is there a specific evolutionary history that underpins the basis of joint lowland and highland residency across a species range, or are there some common ecological factors or life history traits (e.g., canopy versus understorey feeding) that are not directly linked with elevation?
We revisit published phylogeographic studies of African montane and lowland bird species and combine these results with summaries of additional case studies from the literature. We synthesize these data and use the results to discuss the possible origins of disjunct elevational distribution of some African bird species in relation to climate history and a putative common ecological cause.
Study Region and Data
The Eastern Afromontane Biodiversity Hotspot represents a suitable area for studying these questions because of its complex landscapes (Figure 1), where isolated mountains and punctuated chains of montane fault-blocks with patches of montane rainforest occur in a matrix of hot and dry lowland savanna with local patches of semi-evergreen forest in drainage seeps and around springs. This can be in the form of foothill forests, groundwater forests associated with the major floodplains, or forest patches near the coast toward the Indian Ocean (Figure 1). While mountains of Kenya and northern Tanzania, and those along the Malawi Rift, are relatively young and partly of volcanic origin, the Eastern Arc Mountains, which run diagonally across Tanzania from the south-west to the Taita Hills in south-eastern Kenya, consist of ancient basement rock that was uplifted over a long period of time, with the final uplift in the late Miocene (>7 million years ago; Griffiths, 1993). Patches of montane rainforests in the Eastern Arc have been interpreted as remnants of the ancient trans-African super-rainforest (Axelrod and Raven, 1978; Lovett, 1993), which broke up during the late Miocene as a consequence of uplift in central Africa, global cooling (deMenocal, 2004), and a shift to more grass-dominated ecosystems over much of Africa (Vrba et al., 1995; Jacobs et al., 1999; Strömberg, 2011).
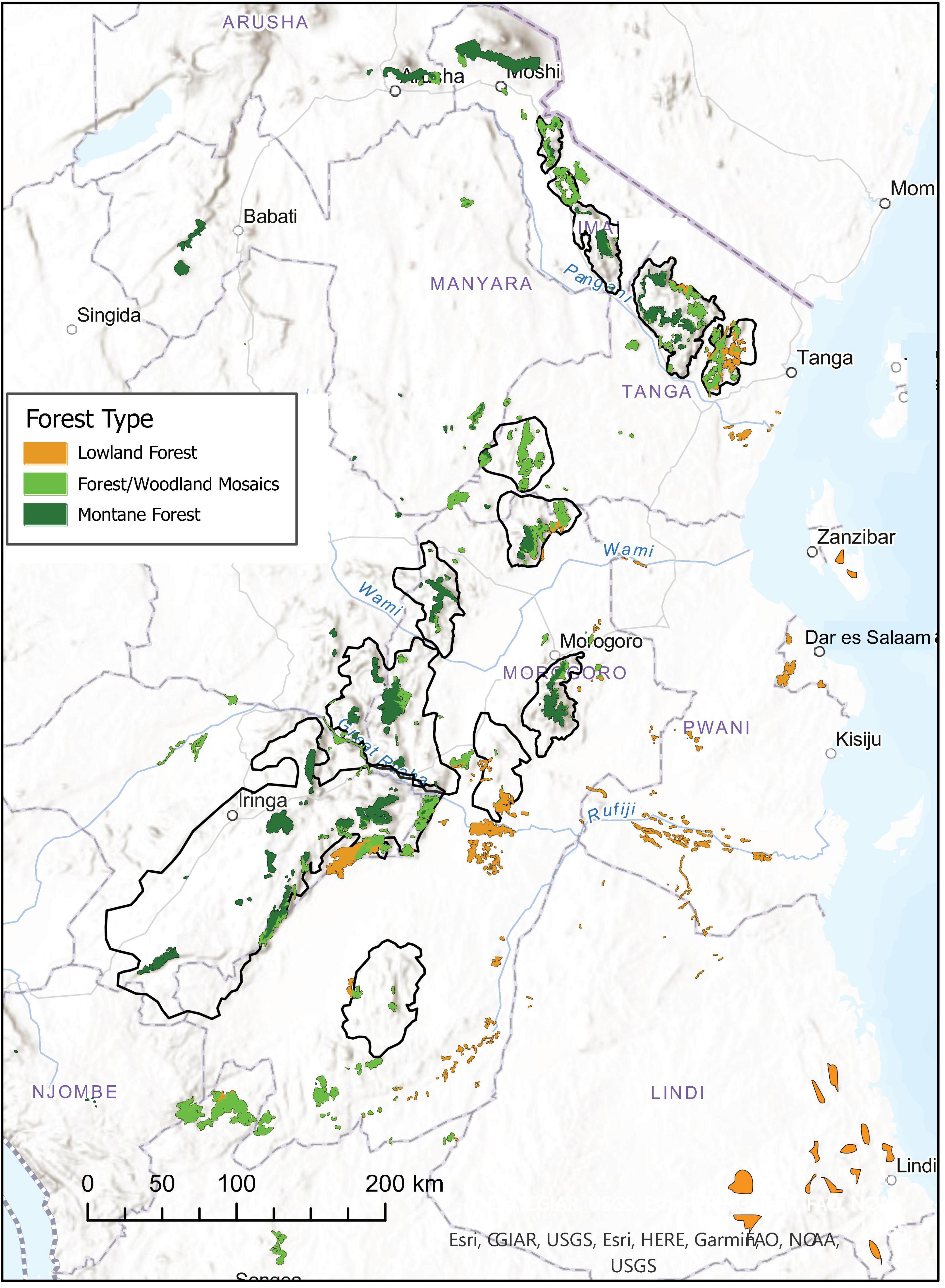
Figure 1. Map of montane and lowland forest fragments distributed across eastern Africa. Black polygons demarcate the boundaries of the Eastern Arc Mountains of Tanzania (see Figure 7). Data compiled as part of the CMEAMF baseline report [Forestry and Beekeeping Division (2006)].
The Eastern Arc Mountains owe their high biodiversity to a predictable supply of humidity from the Indian Ocean, which presumably has been constant since the Miocene (Prell et al., 1980). The eastern escarpments have high orographic rainfall, mainly from November through April. In the East Usambara Mountains, the high humidity from the ocean means that even low hills can maintain cloud forest (Lovett, 1993). The high plateaus further inland are often enshrouded by clouds, which help to maintain soil humidity and therefore the presence of evergreen forest. However, much of the highlands lack forest, because of human activities (burning and clearing for agriculture), or due to low heat-retention in small highlands and frost damage to the vegetation (Sarmiento, 1986; Lovett, 1993). Changes in abiotic factors along the elevational gradient have resulted in distinct bands of vegetation (Lovett, 1993). Mountains along the Malawi Rift are influenced by a more local convection-rainfall, where the rainfall can be somewhat out of pace with the seasonal cycle from the Indian Ocean, and where the climate becomes generally cooler at more southerly latitudes.
The lowlands of East Africa are mainly characterized by savanna woodland and scrubland, with semi-evergreen aspects only locally, and especially along the coastal zone (Burgess and Clarke, 2000). The patches of coastal forest that remain today in the densely populated coastal zone are of quite variable appearance, with little green foliage in the dry season, except in places with high ground water levels near the major rivers, in higher-lying areas with special soils, or in places where fog may accumulate during the night. The understorey is typically dominated by Elyra grasses and has few ferns; screw-palms (Pandanus) can form distinct stands in places with high levels of ground water. There is a flush of green leaves, flowers and insect life during the rainy season, which extends from November to April, or as two annual peaks around equinox as we approach the equator in northern Tanzania and Kenya.
Intensive charting of the distribution of biodiversity has taken place since the 1990s, with comprehensive review of the literature and of material in major museums, and with recent ornithological surveys to virtually every tract of montane forest and also to many lowland forests in Tanzania. These efforts have been supplemented by other recent initiatives, and by bird atlas projects covering much of the region (Dowsett-Lemaire and Dowsett, 2006)1, resulting in detailed distributional databases of species occurrences (Burgess et al., 2006; Rovero et al., 2014). Unfortunately, northern Mozambique is still poorly explored.
Tissue and blood samples of birds for genetic study have been collected in Tanzania and Malawi over the past 20 years by members of several institutions (see “Acknowledgments”) and we make use of some of these samples in our present study.
The results reported in this study are based on Sanger sequencing of mitochondrial markers [NADH dehydrogenase subunit 2 (ND2) and subunit 3 (ND3), Cytochrome b (Cytb), ATP Synthase membrane subunit 6 (ATP6)] and several nuclear introns [e.g., Fibrinogen beta chain intron 5 (FGB5), Glyceraldehyde 3-phosphate dehydrogenase intron 11 (GAPDH 11), Transforming growth factor beta 2 intron 5 (TGFb2)] following standard methods (see Fuchs et al., 2004; Kimball et al., 2009; Bowie et al., 2018). Specific markers used are mentioned with respect to each case study. The loci were aligned using MAFFT (Katoh and Standley, 2013). Phylogenetic trees were built using parsimony in PAUP* (Swofford, 2002) and/or by using maximum likelihood via RAXML v8.2.12 (Stamatakis, 2014) as implemented through the CIPRES supercomputing portal (Miller et al., 2010) under a GTR model of nucleotide substitution; with support for nodes evaluated using bootstrapping. To estimate times of divergence we made use of the rates of molecular evolution calculated by Lerner et al. (2011) for the Hawaiian honeycreeper radiation that we implemented through use of a Bayesian algorithm in BEAST (Drummond and Rambaut, 2007).
Results
We first review published phylogeographic studies of East African bird species with special focus on evolutionary relationships between highland and lowland populations, and for where signs of gene flow between highland and lowland populations have been postulated. In addition, we also mention additional examples of East African bird species whose distribution patterns are suggestive of similar shared histories between montane and lowland habitats, and note that these taxa are in need of phylogeographic analyses.
Cisticolidae, African Warblers: The Artisornis/Oreolais Lineage
The four species comprising the genera Artisornis and Oreolais are insectivorous warblers restricted to the vine-tangles and dense understorey vegetation of montane forests across east and central Africa (Figure 2, Nguembock et al., 2008; Bowie et al., 2018). The African Tailorbird A. metopias is distributed from northern Tanzania to the mountains on the eastern side of Lake Malawi in southern Tanzania (Matengo Highlands) and northern Mozambique (Serra Jeci, near the east bank of Lake Malawi). Its sister-species, the Long-billed Tailorbird A. moreaui has a strange, disjunct distribution with one population on Serra Jeci and another 1,000 km away, in the lower to middle montane forests of the East Usambara Mountains near the coast of northern Tanzania (Figure 2). This distribution pattern has puzzled many biogeographers (e.g., Stuart, 1981).
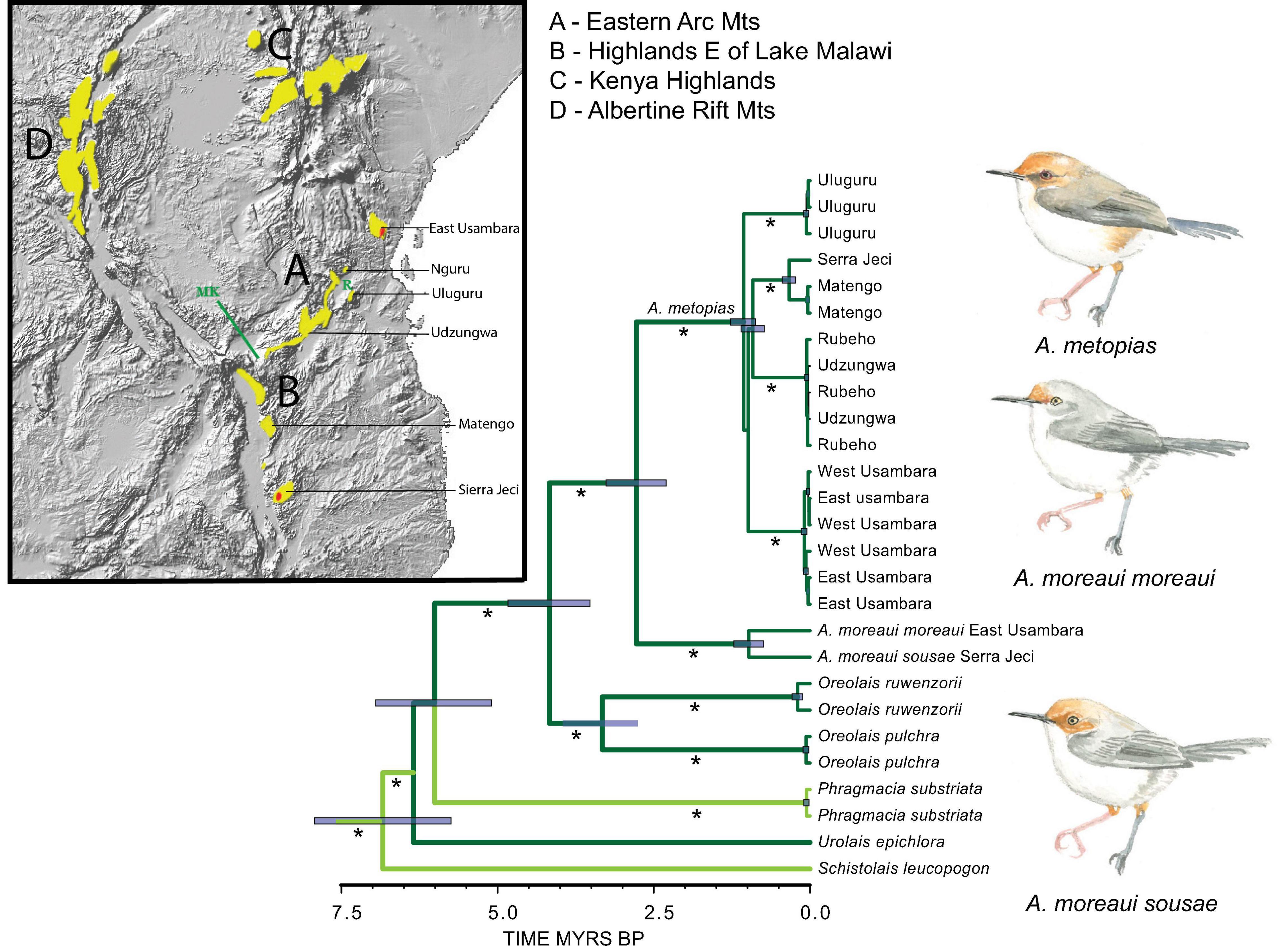
Figure 2. Distributions of Oreolais species in the Albertine Rift (O. ruwenzorii) and Gregory Rift Mountains (O. pulchra) and Artisornis in the Eastern Arc Mountains (A. metopias, and the relict distribution of A. moreaui in red); and phylogeny of these taxa and the deeply divergent Phragmacia of South African arid lowland habitats, and Urolais and Schistolais of the Guineo-Congolian rainforest region. The phylogeny is derived from a maximum likelihood analysis of seven genes using 13 partitions detailed in Bowie et al. (2018). Timing of divergence was estimated using the rates of nucleotide substitution reported by Lerner et al. (2011). On the map: R, Rubeho Mountains; MK, the Makambako Gap delineating the southern extent of the Eastern Arc Mountains. * > 75% bootstrap support. In this phylogeny (and for following illustrations), lineages associated with montane forest are marked with dark green and those in lowland habitats with light green.
Bowie et al. (2018) provided evidence in support of the contemporaneous Pleistocene vicariance of the once continuous range of the African Tailorbird into four distinct clades each reciprocally monophyletic: (1) Usambara Mountains, (2) Central Eastern Arc Mountains, (3) Uluguru Mountains, and (4) the Matengo and Serra Jeci Highlands; Figure 2). The simultaneous separation of the two populations of A. moreaui with that of the divergence of the four populations of A. metopias, suggests that both species were affected at the same time during the Pleistocene, when Africa became more arid (deMenocal, 2004; Trauth et al., 2005; Lyons et al., 2015) and lowland and highland forests were fragmented. To explain the present disjunct distribution of the two extant populations of Long-billed Tailorbird, Bowie et al. (2018) inferred a direct dispersal via lowland forest between the East Usambara Mountains (one of the few places in Africa where montane and lowland forest about) and the forest habitats of northern Mozambique. Molecular data from other montane bird species (e.g., Bowie et al., 2006; Fjeldså et al., 2006; Fuchs et al., 2011) as well as montane frogs (Lawson, 2013; Portik et al., 2019) suggests the existence of such a lowland corridor along coastal Tanzania and possibly across the hilly landscape of southern Tanzania and northern Mozambique. Lowland forests are assumed to have been far more extensive in the past than they are today (Burgess and Clarke, 2000), and therefore A. moreaui could have been more widely distributed through the eastern coastal lowland, but through climate change and anthropogenic modification of the landscapes, the species went extinct in most of the area, leaving behind the disjunct distribution we observe today.
Cisticolidae: The Plain-Backed Duetting Cisticolas
Within the largest genus of songbirds, the cisticola warblers (genus Cisticola), a small group of species with unstreaked dorsal plumage and with duetting songs, have long been recognized as a distinct montane clade with three species (Lynes, 1930; Davies, 2014): Chubb’s Cisticola C. chubbi in the Cameroon Mountains and along the Albertine Rift to western Kenya; Hunter’s Cisticola C. hunteri in the highlands of Kenya and northern Tanzania; and Black-lored Cisticola C. nigriloris in the Rubeho and Udzungwa highlands of Tanzania and the highlands flanking the northern end of Lake Malawi and adjacent Zambia (at 1,100–2,550 m). These Cisticolas inhabit montane forest, scrub, bracken and tall grassy vegetation associated with swamps and seeps (Urban et al., 1997; Ryan, 2006). A fourth member of this clade, the Kilombero Cisticola C. bakerorum, was recently described from the lowland reed-marshes of the Kilombero floodplain in southern Tanzania, a distinct ecological zone at only 240–305 m a.s.l from that occupied by the other three species in the clade (Fjeldså et al., 2021). The Kilombero Cisticola is sister to the geographically neighboring C. nigriloris, and molecular dating methods suggested that these two taxa diverged at the Pliocene-Pleistocene transition (2–3 Mya). Given the montane ancestral state reconstruction for the African duetting Cisticolas, a plausible evolutionary scenario is a down-slope dispersal of the ancestral Kilombero Cisticola that eventually led to the establishment and isolation on an ever-humid floodplain represented today by the lowland expanse of the Kilombero.
Platysteiridae: The Batis Flycatchers
Within the speciose genus Batis, the sexually dimorphic Batis capensis superspecies (Hall and Moreau, 1970) is primarily associated with montane forests and thickets that extend from northern Tanzania, through Malawi, the highlands of northern Mozambique and eastern Zimbabwe to reach the Cape Province in South Africa, where montane forest reaches sea level as latitude compensates for altitude. Traditionally considered a single species with: mixta occupying the costal forests of southeastern Kenya and the mountains of Tanzania and extreme northern Malawi; sola the mountain blocks east of Lake Malawi; dimorpha the mountains of southern Malawi and northern Mozambique; and several closely related taxa occupying the montane (erythrophthalma) and lowland hill habitats (kennedyi) of Zimbabwe and South Africa (capensis and hollidayi). An outlying lowland taxon, reichenowi, inhabits the lowland forests on the Rondo Plateau and other sites in the southeastern corner of Tanzania near the Mozambique border. Most of the populations from northern Malawi through Tanzania inhabit montane forest, although there is evidence of seasonal visits to wooded foothills (down to 540 m) in the dry season (Fjeldså et al., 2006, 2010), and some populations of mixta in the coastal zone of Kenya and northern Tanzania are resident to lowland forest like reichenowi.
A phylogeographic study by Fjeldså et al. (2006) demonstrated that the East Africa taxa (mixta, dimorpha, and sola) do not form a mophophyletic clade, because the Rwenzori Batis B. diops of the Albertine Rift Mountains is more closely related to mixta populations in coastal Kenya and the northern Tanzanian Mountains (extending to the Nguru Mts), whereas the populations inhabiting the highlands of south-western Tanzania are genetically distinct. Fjeldså et al. (2006) recognized the latter populations as a new species, the Dark Batis, B. crypta, which extends from north and east of Lake Malawi through the southern Eastern Arc Mountains to the Ukaguru Mountains in central Tanzania. Species rank for B. crypta was awarded because of reciprocal monophyly, diagnostic morphological characters, and the lack of evidence for gene flow with the Forest Batis B. mixta, although the two species are separated by only 25 km of hills (potential habitat in the non-breeding season) between the Ukaguru and Nguru Mountains. Intriguingly the phenotypically distinct reichenowi, which is isolated in the coastal forests of south-eastern Tanzania, was nested within B. mixta in the molecular phylogeny of Fjeldså et al. (2006) – a result corroborated by our expanded analyses in this paper (Figure 3), with the caveat that due to the lack of tissue samples, we are unable to determine the phylogenetic position of Woodward’s Batis B. fratrum, which somewhat resembles mixta and reichenowi and inhabits the lowlands of Mozambique and southern Malawi.
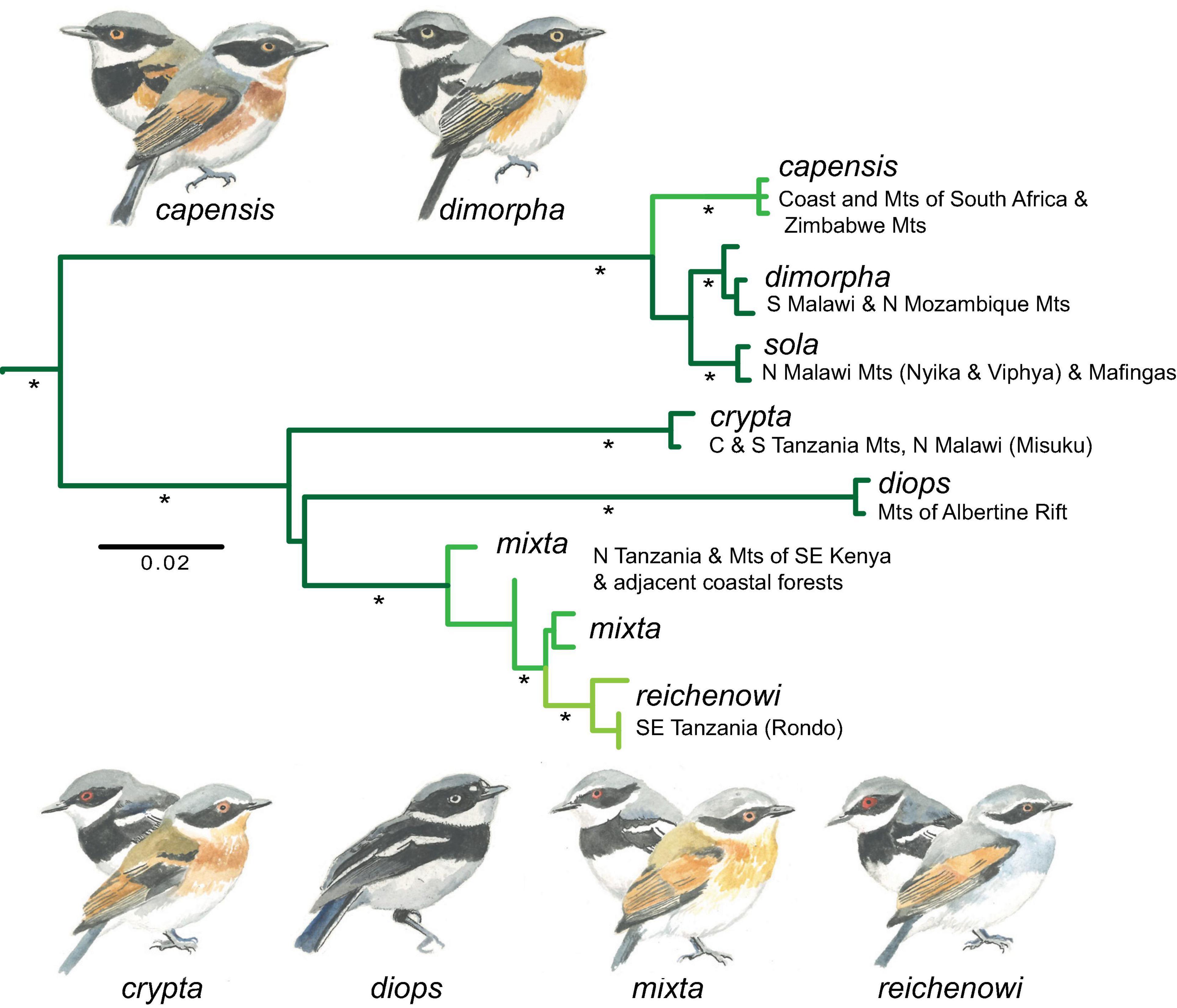
Figure 3. Multilocus DNA (3 loci) phylogeny of Batis species of east and southern Africa derived from a partitioned maximum likelihood analysis. Dark green branches are indicative of lineages that occupy montane forest, light green is indicative of lowland forest, and the intermediate green color is indicative of lineages that occupy a broad elevation range extending into both lowland and montane forest. * > 75% bootstrap support.
In our expanded phylogenetic analysis that now includes populations of Batis distributed across the Malawi Rift and southern Africa (Figure 3), montane populations of the northern sola and southern dimorpha were recovered as monophyletic clades sister to the Cape Batis B. capensis of southern Africa. Similar to the parapatric distribution of B. mixta and B. crypta in the Eastern Arc Mountains, the ranges of B. crypta and B. (capensis) sola are separated by only 10 km of mid-altitude rangeland and palm savanna. The Albertine Rift B. diops is recovered as closely related to the B. mixta/crypta/reichenowi species complex; an interesting result given that B. diops is phenotypically monomorphic compared to all other populations in this study, which are dimorphic.
The geographically isolated population of B. mixta reichenowi (Fjeldså et al., 2006, treated as an independent species by del Hoyo and Collar, 2016) would represent a phylogenetic species (Cracraft, 1983) based on the distinctive appearance of the female plumage (Figure 3). The nested position of reichenowi in the phylogeny suggests that this small satellite population of Batis diverged by budding from the more widely distributed northern B. mixta, and is likely a relict population left after a humid period when coastal forest extended along the east coast of Africa, where genetic drift and rapid fixation of alleles (possibly the MC1R gene affecting expression of melanin; see Harris et al., 2020) resulted in the distinct plumage of reichenowi. The contraction of coastal forest as Africa became more arid through the Pleistocene and associated anthropogenic change likely left reichenowi as a relictual taxon now restricted to some lowland forests in south-eastern Tanzania.
Muscicapidae, Subfamily Cossyphinae: The African Robins
The African robins comprise a monophyletic clade of c. 45 insectivorous species (Fjeldså et al., 2020), with the majority of these species distributed in forest understorey and thickets across sub-Saharan Africa, especially in the lower montane zone (Voelker et al., 2010). The family as a whole, like many other families of “higher songbirds” (Passerida, see Fjeldså et al., 2020), shows a high thermal flexibility and tendency to radiate in montane regions. However, some species of robin-chats (Cossypha, Dessonornis, and Caffrornis) can be found in thickets at all elevations from the lowlands to the treeline, with a few species even penetrating arid savanna. To illustrate the elevational flexibility of these lineages we provide new analyses of two lineages [Sheppardia species (akalats), forest-chats in the genus Chamaetylas], and place these results in context with those from a published study of a third lineage, the White-stared Robin Pogonocichla stellata (Bowie et al., 2006).
The genus Sheppardia includes at least 10 species that form a monophyletic clade within the larger African forest robin assemblage (Voelker et al., 2010; Fjeldså et al., 2020). Akalat species are typically restricted to montane or lowland forest, with a few species (e.g., East Coast Akalat S. gunningi) occupying the entire elevational gradient. The greatest diversity of Sheppardia species occurs within the Eastern Arc Mountains of Tanzania (five species). These five species comprise three clades (Figure 4, see also Voelker et al., 2010). (1) The Usambara Akalat species complex S. lowei-aurantiithorax-montana primarily occupies the interior of well matured forest with dense shrubbery and liana tangles on upland plateaus at 1,400–2,400 m, with species replacing each other across the different montane highlands of the Eastern Arc Mountains (Beresford et al., 2004). (2) Sharpe’s Akalat S. sharpei inhabits forests in the mid-montane zone (1,030–2,160 m) extending through most of the Eastern Arc Mountains, with subspecies usambarae occupying the northern Eastern Arc to the Nguru Mountains, and sharpei the remainder of the Eastern Arc, the volcanic highlands of southern Tanzania and the northern and central highlands of the Malawi Rift. Our present analysis (Figure 4) and that of Voelker et al. (2010) recover Sharpe’s Akalat as sister to a clade comprising the lowland Gray-Winged Akalat S. polioptera and the elevationally variable Bocage’s Akalat S. bocagei, although with relatively weak bootstrap support. (3) S. gunning is mainly found in lowland forest (to 300 m) along Africa’s east coast, with the pale plumaged nominate subspecies gunningi occupying the coastal forests of north-central Mozambique. The subspecies sokokensis occupies the coastal forests of south-eastern Kenya and northern Tanzania, extending to Zanzibar Island. In contrast, the remaining two subspecies, which are more richly pigmented (Figure 4), are found in the lower montane zone; bensoni in northern-central Malawi and on Mount Mabu in northern Mozambique (500–1,750 m), and alticola on the steep Nguu Mountains (alias Nguru North) in Tanzania (850–1,750 m, Fjeldså et al., 2010). S. gunningi and S. sharpei do not overlap geographically: this results in the northern alticola and southern bensoni having disjunct ranges >600 km apart, and hence, it is surprising that they form a monophyletic clade (Figure 4) to the exclusion of more geographically proximate taxa (sokokensis and gunningi, respectively). This result lends further support to a past long-distance connection between northern Tanzania and northern Mozambique, consistent with interpretation of phylogeographic structure in the Artisornis warblers as described above (see also Bowie et al., 2018).
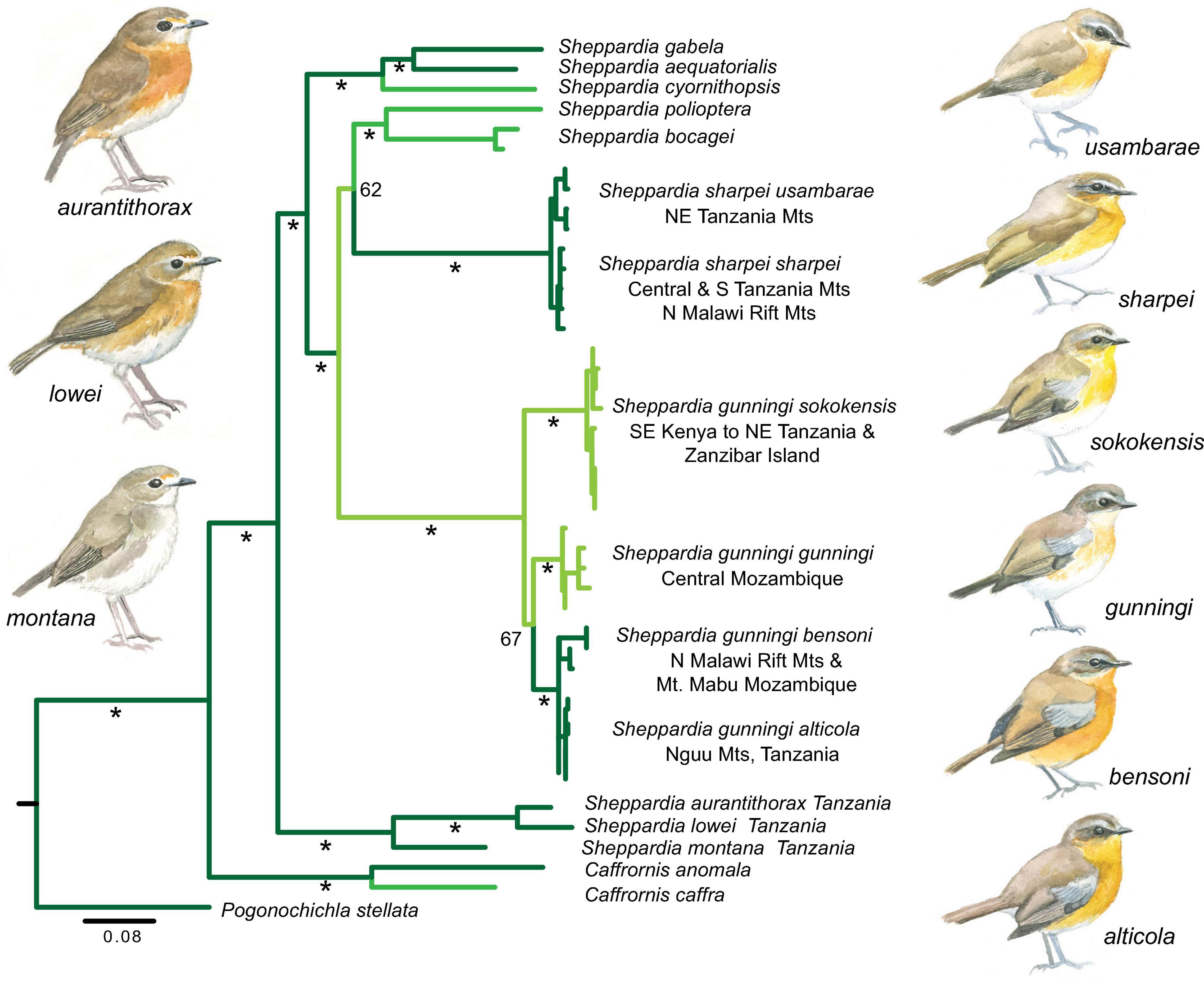
Figure 4. Mitochondrial DNA (ATP6, ND2) phylogeny of Sheppardia akalats of Africa derived from a partitioned maximum likelihood analysis. Dark green branches are indicative of lineages that occupy montane forest, light green is indicative of lowland forest, and the intermediate green color is indicative of lineages that occupy a broad elevation range extending into both lowland and montane forest. * > 75% bootstrap support.
The White-Starred Robin Pogonocichla stellata is one of the most widespread Afromontane birds, occupying highlands around the montane circle of Africa, with its range extending through the Malawi Rift to the Western Cape in South Africa. Periods of aridity during the early- to mid-Pleistocene resulted in regional structuring of populations with breaks in gene flow separating populations in: (1) the Albertine Rift (ssp. ruwenzorii); (2) Kenyan Highlands (keniensis); (3) the northern Eastern Arc (helleri); and (4) central Eastern Arc and Malawi Rift mountains (Nguru to northern Mozambique; ssp. orientalis) (Bowie et al., 2006). Some sharing of mitochondrial DNA haplotypes occurs between ruwenzorii and orientalis, and between helleri and orientalis, with coalescent modeling suggesting that the sharing of haplotypes by ruwenzorii and orientalis is due to ancestral polymorphism rather than recurrent gene flow (Bowie et al., 2006). In contrast, gene flow seems to be taking place between the montane populations of northern Tanzania (helleri) and southern Malawi (orientalis) to the exclusion of the interior Eastern Arc Mountains and the central and northern Malawi Rift. It is likely that the White-starred Robin undergoes seasonal elevational migration with the coastal forests of Tanzania and northern Mozambique providing a “corridor” of connectivity, in a similar manner as has been inferred for the Long-billed Tailorbird and East Coast Akalat mentioned in the preceding case studies.
The chunky forest chats in the genus Chamaetylas inhabit the dark forest understorey, often foraging near swarms of driver ants (Dorylus), but in the dry season they are also found in riparian forests in the adjacent foothills (Fjeldså et al., 2010). Surprisingly, small/localized breeding populations, which have been classified as C. fuelleborni xuthura (Clancey and Lawson, 1969) have been found much further south, in lowland sand forests between Beira and the Zambezi River in Mozambique. Records of Chamaetylas forest chats from a coastal forest in southeastern Tanzania could be seasonal migrants from the Eastern Arc Mountains, but they could also represent a resident population and an outlier from the Mozambique lowland population (Jensen et al., 2005). Due to the presence of landmines, many parts of northern Mozambique remain difficult to explore, hence, the forest patches that occupy the hills and inselbergs of northern Mozambique are largely unknown.
The distribution of the White-chested Alethe Chamaetylas fuelleborni, with nominate fuelleborni occurring in the montane highlands of the Eastern Arc Mountains and the mountains flanking the northern Malawi Rift, and the form xuthura of the lowland forests in Mozambique, point to a past connection through the once more extensive coastal forests of Tanzania and Mozambique, probably reaching the Lebombo Mountains south of Maputo. We presently lack molecular data for xuthura, but should this taxon be sister to the montane Thyolo Alethe C. choloensis that occupies the mountains of southern Malawi, instead of C. fuelleborni, this would still illustrate extraordinary elevational flexibility in these forest chats.
Pellorneidae, the Jungle Babblers
The genus Illadopsis comprises eight insectivorous species mainly inhabiting understorey habitats in the Guineo-Congolian rainforests. However, Pale-breasted Illadospis Illadopsis rufipennis has a sister species, Mountain Illadopsis I. pyrrhoptera, in the montane forest of the Albertine Rift and an isolated population in northern Malawi. Small local populations of Illadopsis in Tanzania have traditionally been referred to as subspecies (distans or puguensis) of I. rufipennis), Molecular phylogenetic analysis places the Tanzanian populations as a sister-group to the montane pyrrhoptera, and the West African I. rufipennis populations as sister to this clade. Hence, the traditional I. rufipennis was not monophyletic (see also Nguembock et al., 2009) and the Tanzanian populations are now recognized as a separate species I. distans (del Hoyo and Collar, 2016). Further, a high degree of phylogeographic structure is recovered among the Tanzanian populations sampled (Figure 5), with deep genetic divergence (5–7% uncorrected) suggesting that these populations started to diverge over the past 2 Mya. These populations occupy both lowland and montane forest, and are subtle morphologically different.
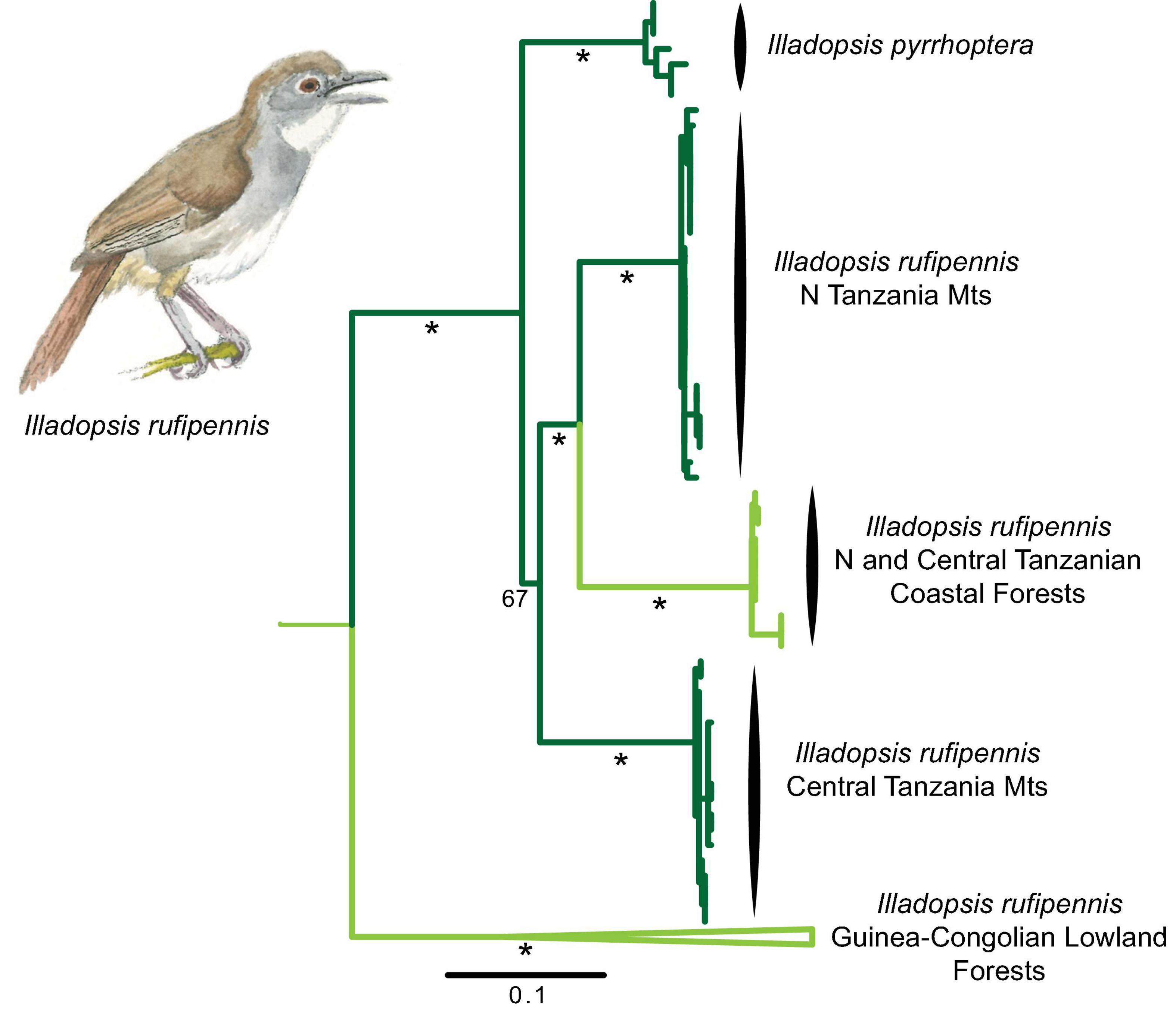
Figure 5. Mitochondrial DNA (ATP6, ND2) phylogeny of Illadopsis species of east Africa derived from a partitioned maximum likelihood analysis. Note that I. pyrrhoptera renders I. rufipennis polyphyletic. Dark green branches are indicative of lineages that occupy montane forest, intermediate green is indicative of lineages that occupy a broad range of elevations and light green lineage occupies lowland forest. * > 75% bootstrap support.
One Tanzanian lineage (nominate distans) is found in montane forest in the Usambara Mountains and further inland in foothills of the Nguru Mountains and adjacent Mount Kanga. Another lineage (still unnamed) is distributed locally in the Rubeho and Udzungwa Mountains, mainly occurring in shady places along forest streams up to 2,000 m, but locally (or seasonally) also in adjacent foothill forests (Fjeldså et al., 2010). A third lineage (spp. puguensis) is found in some lowland forests near Dar es Salaam and further south near the Rufiji Delta, and genetically similar birds are found in groundwater forests in the foothills of the Uluguru Mountains 160 km inland, and (surprisingly) in montane forest in the Kiboriani Mountains. A small lowland population on Zanzibar Island has not yet been included in molecular phylogenetic analysis. These populations are highly fragmented and are of critical conservation concern.
Pycnonotidae, Greenbuls of the Genus Phyllastrephus
This group, with at least 18 insectivorous species, is mainly distributed in lowland gallery forests with dense vine-tangles and epiphytes. Adaptation to higher elevation occurs among members of the Yellow-streaked Greenbul Phyllastrephus flavostriatus complex, in Cabanis’s Greenbul P. cabanisi and Placid Greenbul P. placidus, and in two local populations of the Tiny Greenbul (see below). Further, the Gray-olive Greenbul P. cerviniventris of central and eastern Africa is mostly associated with riparian and groundwater forest in montane foothills, but it is also found locally in small riparian thickets or forest swamps up to 1,900 m in Malawi and Tanzania (e.g., Dowsett-Lemaire and Dowsett, 2006; Fjeldså et al., 2010; John and Kiwango, 2021).
Phyllastrephus debilis, which has an isolated position in the phylogeny of the genus (Johansson et al., 2007; Fjeldså et al., 2020), is found across the mosaic of coastal forests in eastern Africa, with subspecies debilis occurring in central Mozambique, and subspecies rabai through the coastal zone of Tanzania and southern Kenya. Distinctly larger birds with more saturated plumage colors inhabit montane forests between 600 and 2,150 m in the West Usambara and Nguru Mountains of Tanzania (ssp. albigula). A multilocus study of 124 specimens from all parts of the range placed debilis and rabai close together but revealed that the albigula populations had been genetically isolated in their montane forest habitats since 2.4–3.1 Mya (Fuchs et al., 2011). The montane-lowland populations are now in secondary contact in Tanzania, as rabai has expanded inland and reaches the lower montane forest on the slopes of some Eastern Arc Mountains. This has resulted in limited recurrent gene flow from rabai to albigula, but the populations appear to have retained their integrity and are now recognized as separate species (Fuchs et al., 2011; Gill et al., 2021). The molecular data could not support or reject the possibility of ongoing gene flow between the montane populations of albigula on the Nguru and Usambara Mountains, which are presently isolated by 125 km of dry lowland plains.
Phyllastrephus flavostriatus is phenotypically and genetically complex. A phylogeographic study based on 248 specimens representing nearly every known allopatric population and using two mitochondrial markers (Lokugalappatti, 2011), placed populations in montane forests distributed across the Albertine Rift (subspecies graueri, olivaceogriseus, itombwensis, and kungwensis) as the sister clade to populations inhabiting variable elevations in southern and eastern Africa; we recover similar results in our analyses (Figure 6). A distinct morphological form (ssp, alfredi, with a brown instead of gray head) inhabits highlands along the Rukwa and Malawi Rifts and may be the first diverging lineage within the Yellow-streaked Greenbul complex, or may form a clade sister to a complex of populations in the Udzungwa Mountains (uzungwensis) and mountain ranges and foothills extending from southern Malawi to the KwaZulu-Natal Province of South Africa (ssp. vincenti and flavostriatus; Lokugalappatti, 2011). Nested among these southern populations are populations (spp. tenuirostris) that inhabit coastal forests from northern Mozambique to southern Kenya, with individuals ascending to montane forest in the northern Eastern Arc Mountains. These data suggests an origin in montane habitats and development of a broad ecological niche during a Pleistocene expansion toward southern Africa, with a final dispersal through the coastal forest zone of East Africa to the adjacent montane forests of the northern Eastern Arc Mountains.
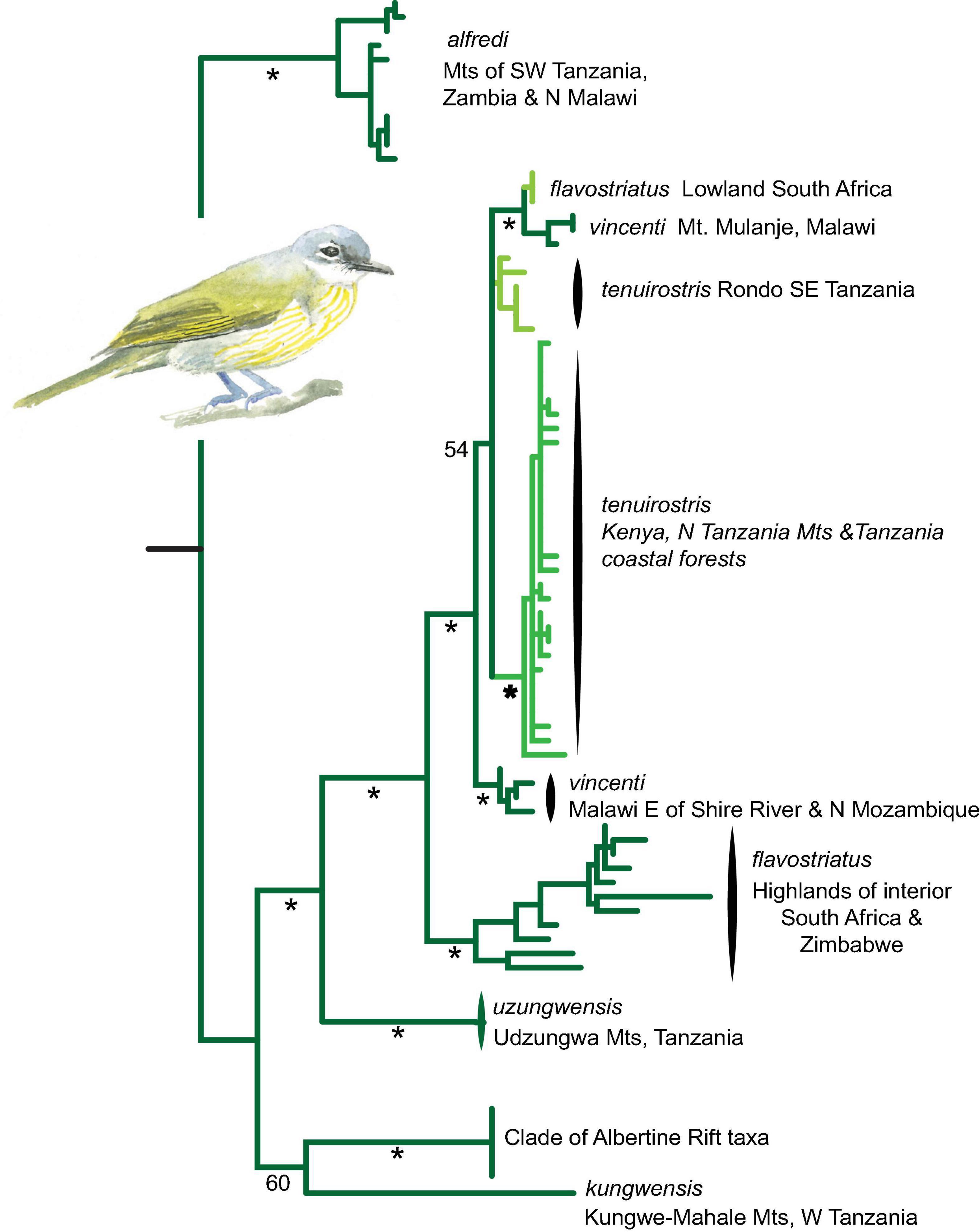
Figure 6. Mitochondrial DNA (ND2) phylogeny of Yellow-streaked Greenbul Phyllastrephus flavostriatus populations across east and southern Africa derived from a partitioned maximum likelihood analysis. Dark green branches are indicative of lineages that occupy montane forest, light green is indicative of lowland forest, and the intermediate green color is indicative of lineages that occupy a broad elevation range extending into both lowland and montane forest. * > 75% bootstrap support.
Lybiidae, African Barbets
This family of fruit-dependent birds is widespread in African lowland forest and woodlands. The Green Barbet Stactolaema olivacea, with three widely disjunct subspecies primarily restricted to lowland forest (olivacea, woodwardi, and hylophona; Figure 7) and three subspecies restricted to montane forest (belcheri, rungweensis, and howelli), provides an ideal exemplar taxon with which to explore speciation patterns among lowland and montane forests, as well as the age and extent of connectivity between these habitats. Particularly intriguing is Woodward’s Barbet (C. olivacea woodwardi), that occurs only in the Ongoye (Ngoye) Forest in KwaZulu-Natal, South Africa, over 2,000 km from its nearest neighboring population in southern Malawi (belcheri). Due to the sharing of bright yellow-green ear coverts, the taxa woodwardi and hylophona from coastal forests in south-eastern Tanzania (Jensen et al., 2005), are traditionally placed as sister taxa (Clancey, 1989) and are often lumped together as the same species (Sinclair and Ryan, 2010).
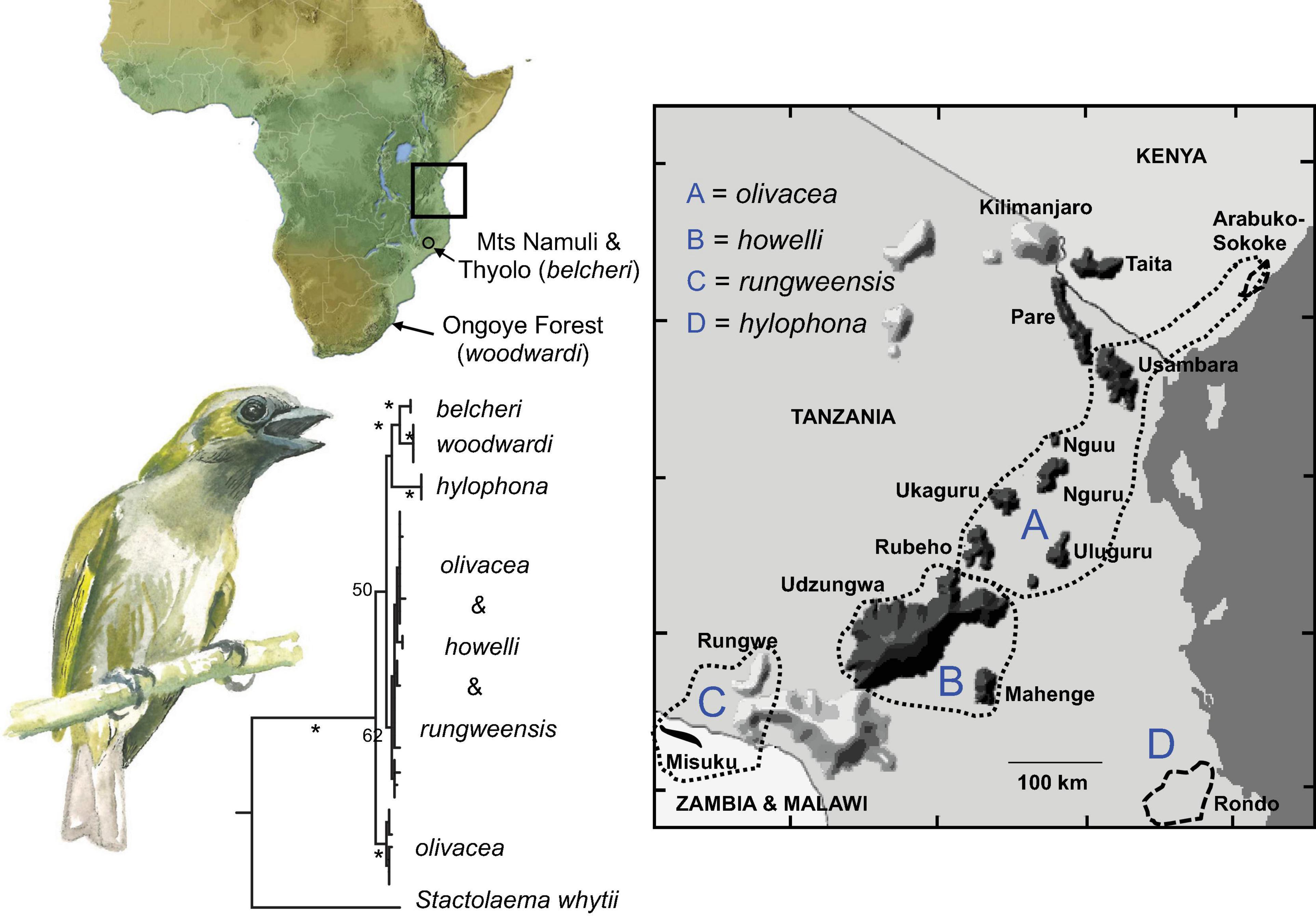
Figure 7. Top Left Panel: Distribution map of the Green Barbet Stactolaema olivacea. Right Panel: An enlargement of the box demarcated on the map of Africa depicting the distribution of Green Barbet subspecies across Tanzania and extreme northern Malawi. Bottom Left: Mitochondrial DNA (Cytb, ND3) phylogeny of Green Barbet populations across east and southern Africa derived from a partitioned maximum likelihood analysis. * > 75% bootstrap support.
Our molecular analyses reveal remarkably shallow sequence divergence among all six subspecies of Green Barbet (max. 2.36%) despite the very large distances separating disjunct populations (Figure 7). This shallow sequence divergence makes the relationship among subspecies difficult to resolve. Three clades are recovered in our molecular phylogenetic analyses. (1) A clade of individuals sampled from the lowland Sokoke Forest along the coast in southeastern Kenya and individuals sampled from the montane forests of the East and West Usambara Mountains (subspecies olivacea). (2) A clade of individuals occupying the montane highlands of the remainder of the Eastern Arc Mountains and the Misuku Hills in extreme northern Malawi (subspecies howelli and rungweensis). (3) A clade comprising three widely disjunct taxa, with woodwardi (lowland) and belcheri (montane) recovered as sister-taxa, and hylophona (lowland) putatively sister to these two taxa. These data point to at least two instances of montane to lowland transitions, suggesting a recent history of dispersal between lowland and montane habitats for this canopy feeding bird.
Additional Putative Cases of Montane-Lowland Range Dynamics in East African Birds
In order to underscore that flexibility in elevational distribution is not just a rare anomaly, we mention below some further cases, which have not yet been adequately evaluated by phylogeographic methods. The many cases of montane species that are nested within larger clades of lowland bird species, provide evidence of past flexibility in elevational distribution.
Superfamily Sylvioidea, “Warblers” in the Broader Sense
Colorful species in the genus Apalis are mainly distributed in canopies of montane forest areas in Central Africa (A. personata, binotata and jacksoni) and the outlier highlands of Cameroon and Angola (A. binotata and jacksoni), with one distinctive species, the White-winged Apalis A. chariessa, with a relictual distribution in East Africa. One population of White-winged Apalis is found in mid-elevation rainforest in southern Malawi (Dowsett-Lemaire and Dowsett, 2006), another within the humid montane forests of the Udzungwa and Uluguru Mountains, and the species also once occurred (at least until 1961) 570 km further north, in the lowland forests of the lower Tana River delta near the Kenya coast. Another interesting case is the Black-headed Apalis Apalis melanocephala, which is patchily distributed from northern Mozambique and from Malawi to Kenya, mainly in highlands but locally also in foothills and coastal forests. Examining material in several museum collections, JF found significant variation in plumage melanization and tail length, both within and between local populations. Age-related variation and polymorphism could play a role as it does in Cisticolas, but there are also signs of introgression between populations in the south (subspecies tenebricosa) and between populations in Tanzanian coastal forests or mountains in the northern part of the Eastern Arc Mountains (ssp. moschi).
Grass warblers of the genus Bradypterus are patchily distributed across sub-Saharan Africa, mainly occurring within the humid undergrowth of montane forests, or in swamp habitats (Kahindo et al., 2017). The Little Rush-warbler, B. baboecala, is genetically structured with local populations both in highlands and lowlands in central and eastern Africa (unpubl. data).
White-eyes, genus Zosterops, represent one of the most remarkable avian cases of rapid radiation in the Pleistocene. Although most of the diversity is found in the Indo-Pacific archipelago, one lineage colonized Africa and diversified throughout the sub-Saharan continent (Cai et al., 2019; Gwee et al., 2020). Recent molecular work suggest that the many populations, which are characterized by broad, white eye-rings and replace each other in different Afromontane areas, do not represent a monophyletic clade but are interspersed in the phylogeny among generally more widespread lowland forms (Martins et al., 2020). This suggests that numerous local populations need to be recognized as independent species (Pearson and Turner, 2017). The results could also be interpreted to suggest several independent cases of speciation along local elevational gradients (Cox et al., 2014), but, given uncertainties about the phylogenetic resolution and remaining gaps in the geographical sampling, it is difficult to exclude the alternative interpretation, that this “island speciator” diversified rapidly across Africa’s “sky archipelago,” to subsequently disperse and settle in the surrounding lowlands.
Nectarinidae, Sunbirds
The sunbirds of Africa have mainly radiated in upland savannas and mountain regions (Bowie, 2003). Recent phylogenetic work has revealed a monophyletic group, Euchloridia (Bowie and Fjeldså, 2020), which is highly heterogeneous in plumage characters, bill shape and diet, resulting in its constituent species having previously been placed in different genera, which we refer to below to avoid confusion. This group appear to represent relict populations from the time when the Miocene evergreen forests extended across tropical Africa from west to east. Most constituent species are dull olive-green birds of lowland rainforest (Deleornis and Anthreptes), but the Banded Green Sunbird Anthreptes rubritorques is a rare inhabitant of mid-elevation forest on some Eastern Arc Mountains. The more colorful East African species Plain-backed Sunbird Anthreptes reichenowi occupies coastal forests, the phenotypically aberrant Rufous-winged Sunbird Cinnyris rufipennis is restricted to wet highland forest in the Udzungwa Mountains and its sister-species, the Amani Sunbird Hedydipna pallidigaster, has a peculiar, patchy distribution, with a small population in the Udzungwa highland forest, another in the East Usambara Mountains, and yet another in coastal forests in Kenya, mainly in Arabuko Sokoke Forest (Fjeldså et al., 2010). These three populations appear to be genetically closely related (unpubl. data) but inhabit very different climates. Thus, even within this relatively old clade with deep divergence between constituent species, we see mixed occupancy of lowland and montane habitats, and elevational flexibility among populations within one species.
The Olive Sunbird Cyanomitra olivacea (now Haagneria; Bowie and Fjeldså, 2020) one of Africa’s most widespread songbirds occupies both montane and lowland forest throughout its range. Analyses by Bowie et al. (2004) of mitochondrial DNA sequence variation across the species range revealed that that birds sampled from the northern Eastern Arc (Taita Hills, Pare, Usambara, and Nguru Mountains) share alleles from the same haplotype clusters as birds from southern Malawi (Mt. Zomba) and northern Mozambique (Mt. Namuli) to the exclusion of the interior Eastern Arc Mountains (Uluguru, Rubeho, and Udzungwa). This suggests that lowland coastal forests along Africa’s east coast have served as a corridor linking coastal forests in the north with those in extreme southeastern Tanzania, and Mozambique, in a similar manner as has been inferred in several of the case studies mentioned above.
Other potential cases of widespread montane forest birds with local populations in coastal forests comprise Eastern Bronze-naped Pigeon Columba delegorguei, Lemon Dove Columba larvata, Silvery-cheeked Hornbill Bycanistes brevis and Black-fronted Bush-shrike Chlorophoneus nigrifrons. The green turacos, the Tauraco persa group, present a very complex case with diverse distributions, occupying both montane and lowland habitats, and would present a very interesting case for detailed phylogeographic study.
A Concise Summary of Our Findings
Our data analyses reinforce earlier views (e.g., Moreau, 1966) that the distribution of forest birds across East Africa is complex. However, through the synthesis of the above case studies we are able to identify some repeated patterns. First, there are some bird species with populations in the Eastern Arc Mountains and in adjacent lowland forests or in the northern coastal forests (Cisticola, Illadopsis, Phyllastrephus albigula/debilis, Zosterops, and Hedydipna pallidigaster). Second, several species distributed in the branch of the Eastern Afromontane Biodiversity Hotspot located in Malawi, or in the lowlands of Mozambique, also have resident populations in the northern coastal forests or in adjacent mountains in the northern Eastern Arc to the exclusion of the central Eastern Arc Mountains (Artisornis moreaui, Batis spp., Sheppardia gunningi, Phyllastrephus flavostriatus, Stactolaema olivacea, and Apalis spp.), and there are indications of gene-flow between these areas (Pogonocichla stellata, Cyanomitra olivacea). Third, some of the populations appear to be relictual, with several montane species nested within clades of mainly lowland species (see Fjeldså et al., 2020 for comprehensive species phylogenies) and vice versa (e.g., Batis spp.). If we take into account also the many bird species with mixed elevational distributions, which have not yet been included in detailed phylogeographic studies, we judge that approximately 15% of all forest-dependent and forest-associated species of East Africa have mixed elevational distributions presently.
Flexible elevational distributions are mainly seen among birds of the forest understorey or mid-canopy, and most of the species are insectivorous. This is for instance the case with the Phyllastrephus greenbuls, while other greenbul genera, which have mixed (insect/fruit) diets, are mainly associated with lowland forest, or specialized to live in montane forest (Arizelocichla). White-eyes and sunbirds have mixed diets (insects and nectar) and Stactolaema is fruit-dependent.
Discussion
The flexibility in elevational distributions of birds in East Africa is quite distinct from what is described for avifaunas of larger and more connected montane regions at low latitudes, such as the Sino-Himalayan Mountains (Päckert et al., 2012) or the South American Andes, where lineages tend to evolve through geographical isolation within narrow elevational bands, with segregation into different ecological zones coming secondarily, by ecological segregation of independent and competing species (e.g., Cadena et al., 2012; Cadena and Cespedes, 2020; Linck et al., 2021).
The rather fuzzy African situation could possibly be interpreted as a consequence of the nature of African mountains as isolated enclaves, or “sky islands” (Bowie, 2003; Fjeldså and Bowie, 2008; Voelker et al., 2010). Most of the African mountains are only moderately high (<2,500 m), and given the instability of the African climate and high seasonality of the intervening savanna matrix habitats, the existence of habitats that would facilitate dispersal of montane birds across lowland habitats may have fluctuated considerably, on timescales from decades through millennia (Nicholson, 2000), and especially through the higher-amplitude Pleistocene glacial periods (deMenocal, 2004; Trauth et al., 2005). This instability, where suitable habitat may not have persisted through climate cycles in some montane highlands (Bowie, 2003), suggests that in order for many African forest birds lineages to survive, they had to exhibit considerable flexibility in their use of different ecological zones. Some species survive only as relict populations in those highlands where remnants of the ancient African rainforest environment had been maintained over evolutionary time (Voelker et al., 2010). Some birds may have been able to survive through flexible use of food resources, for instance by being able to shift their diets between fruits, grain and insects, or by using different microhabitats (interior forest vs. treefall gaps or edges). However, some of the specialized insectivores appear to be sensitive and must leave their breeding habitat during the dry season (Mulwa et al., 2012)2. Most of our cases of flexible elevational distributions are insectivores. Little is known about where birds move in the dry season, but casual observations in Tanzania of highland birds in riparian habitats in the montane foothills or in near-by patches of lowland forest suggest that most birds do not move far from their highland breeding habitat (Burgess and Mlingwa, 2000; Bowie et al., 2006), although still far enough to sometimes end up in the neighboring sky islands on their return migration.
Seasonal elevational migrations could lead to establishment of resident populations in lowland sites that are hydrologically stable, as is the case for Cisticola bakerorum highlighted above, and the case described for Phyllastrephus debilis suggests that lowland lineages can also adapt to occupy montane forest. When moving between cool highlands and hot lowlands, the birds will have to adapt to markedly different temperature regimes, although some groups may be thermally flexible (Khaliq et al., 2015) and other factors, such as soil humidity, amount of green foliage and local abundance of insects could be more important than temperature. The existence of breeding populations in Arabuko-Sokoke forest in coastal Kenya of some birds that otherwise inhabit montane forest could simply reflect the large extent of this forest, which allows it to harbor viable populations. The complex distributions of highland and lowland forms of, for instance, Illadopsis and Zosterops, suggests that shifts between different elevational climates may not be a significant challenge for these lineages. Based on the molecular data presented here, most of the elevational shifts took place during the Pliocene and Pleistocene, when Africa’s climate was overall unstable, as documented from analyses of pollen in cores drilled from lake bottoms (Lyons et al., 2015), which indicate that rainfall regimes varied extensively across the continent (Trauth et al., 2005), with abnormally dry conditions during glacial periods (Lyons et al., 2015).
Above we presented several independent cases of connections between the mountains along the Malawi Rift or the adjacent Zambezian savanna region and the northern section of the coastal forests mosaic (including montane habitat islands in the northern part of the Eastern Arc Mountains), to the exclusion of the resident populations in the central and western Eastern Arc Mountains. This is manifest as indications of gene flow as well as cases of apparent long-distance vagrancy and establishment of highly disjunct distributions, as seen in Artisornis moreaui and Sheppardia gunningi. Given the number of such cases, this appears to be a repeated pattern, which could reflect one or more past connections across the lowland habitats of East Africa. Genetic indications of past range fragmentation in Pogonocichla stellata and Cyanomitra olivacea corresponds to the start of cooling of the northern biomes from the late Pliocene (Bowie et al., 2004, 2006).
We can assume two different scenarios for connectivity between the northern Eastern Arc and southern Malawi Rift. Some species may have been more widespread across forested landscapes in northern Mozambique and southern Tanzania, for instance in extensive bamboo forests or in patches of evergreen forest associated with inselbergs. As Africa became arid, lowland forests retreated and once-connected populations become fragmented, leaving remnant populations in the south and north (e.g., Artisornis, Bowie et al., 2018), and eventually also in south-eastern Tanzania [Batis (mixta) reichenowi, Stactolaema olivacea hylophona].
Another possibility is that birds breeding in the south (Malawi Rift, Mozambique, or Zambezian savanna region) migrated because of seasonal dryness, to reach “wintering” areas in the northern coastal forests. The coastal zone of northern Mozambique is in the rain shadow of Madagascar, and therefore does not receive the same predictable rainfall as the northern coastal forests and the montane forests of the Eastern Arc Mountains of Tanzania. The mountains of the Malawi Rift are influenced by local convectional rainfall cycles, which has been more variable over time than that of the Eastern Arc Mountains (Lovett, 1993). Conditions may therefore have been unstable over time, and in order to maintain breeding populations in the south, the forest birds would have to be ecologically flexible with vagrancy or seasonal migrations becoming an integral part of their life cycles (confer Winger et al., 2018). It is important in this context to note that the rainfall in the northern part of the coastal zone is concentrated around the equinoxes, with peak rainfall in April–May, and a weaker peak in October–November. This means that this zone is still lush and green, with abundant insect life, when Mozambique has its dry season, and this cycle of rain in the northern coastal zone is assumed to have been stable over evolutionary time (Prell et al., 1980; Marchant et al., 2007; Mumbi et al., 2008). This provides opportunities for birds breeding in the south to migrate north, where they may have been able to settle and breed in suitable places with a more predictable rainfall in the northern part of the Eastern Arc Mountains. The establishment of new populations through flexibility in migratory systems is analogous with recent (re)interpretations of other migratory systems, such as the rapid establishment of Nearctic migratory groups throughout the Neotropics (Winger et al., 2014) or the establishment of Sylvia warblers (of Palearctic origin) across Africa through migratory dropoff (Voelker et al., 2009; Voelker and Light, 2011).
In summary, we suggest that the extent of interactions between montane and lowland bird communities in East Africa has been underestimated. We present phylogenetic data that demonstrate how elevational flexibility has likely been selected for over evolutionary time and hypothesize that seasonal altitudinal migration between montane and lowland habitats is not only important at ecological time scales, but has likely played a role in facilitating the diversification of East Africa bird species. We urgently need to apply new methods of animal tracking East African forest bird communities in order to better understand how and when montane and lowland bird species move among habitat patches.
Data Availability Statement
This study forms a synthesis of primarily previously published research, with updated analyses of these data. Molecular data are available on GenBank and were drawn from the following publications: Beresford et al. (2004), Bowie et al. (2004, 2006, 2018), Fjeldså et al. (2006, 2021), Nguembock et al. (2009), Voelker et al. (2010), Lokugalappatti (2011), with GenBank numbers provided in these publications.
Ethics Statement
This animal study was reviewed and approved by the University of California, Berkeley (IACUC R317, 2014-10-6780, 2016-04-8665).
Author Contributions
JF made all paintings of birds used in the figures. Both authors contributed to the article and approved the submitted version.
Funding
This study was supported by funds from the Center for Macroecology, Evolution and Climate at University of Copenhagen, University of California, Berkeley, DST-NRF Centre of Excellence at the FitzPatrick Institute of African Ornithology, and Skye Foundation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the very many people who have participated with us in the field, as well as those who have contributed data to databases and deposited samples for molecular analyses in museums. We also thank the following museums for loans of samples for molecular analyses: Field Museum of Natural History; Burke Museum, University of Washington; Museum of Vertebrate Zoology, University of California, Berkeley; Natural History Museum of Denmark, Peabody Museum, Yale University; Louisiana State University Museum of Natural History; National Museums of Malawi; National Museum of Kenya; United States National Museum; British Museum of Natural History; American Museum of Natural History; FitzPatrick Institute of African Ornithology; KwaZulu-Natal Museum; Muséum National d’Histoire Naturelle (Paris); and Museum of Zoology, University of Michigan. Michael Lawes is thanked for a some Green Barbet samples, Phil Clarke is thanked for viewpoints concerning East African coastal forests, and Jonathan Green and Neil Burgess are thanked for contributing the GIS shape files that were used to construct Figure 1. Finally, we thank Bill Monahan for help constructing Figure 1.
Footnotes
- ^ http://tanzaniabirdatlas.net/start.htm
- ^ Dinesen, L., Lehmberg, T., Romdal, T. S., Sonne, J., and Hansen, L. A. (in review). Seasonal change in bird species community in the Udzungwa Mountains – an Afromontan evergreen forest in Tanzania. Front. Ecol. Evol.
References
Axelrod, S. I., and Raven, P. H. (1978). “Late Cretaceous and Tertiary vegetation history of Africa,” in Biogeography and Ecology of Southern Africa, ed. M. J. A. Werger (Junk: The Hague), 77–130.
Beresford, P., Fjeldså, J., and Kiure, J. (2004). A new species of akalat (Sheppardia) narrowly endemic in the Eastern Arc of Tanzania. Auk 121, 23–34.
Bowie, R. C. K. (2003). Birds, Molecules and Evolutionary Processes among Africa’s Islands in the Sky. Ph.D. thesis. South Africa: University of Cape Town.
Bowie, R. C. K., and Fjeldså, J. (2020). “Superfamily Passeroidea. Introduction and the early lineages,” in The Largest Avian Radiation, eds J. Fjeldså, L. Christidis, and P. G. P. Ericson (Barcelona: Lynx Ed), 263–275.
Bowie, R. C. K., Fjeldså, J., Hackett, S. J., Bates, J. M., and Crowe, R. M. (2006). Coalescent models reveal the relative roles of dispersal, vicariance and ancestral polymorphism in shaping phylogeograpnical structure of an African montane forest robin. Mol. Phylogenet. Evol. 38, 171–188. doi: 10.1016/j.ympev.2005.06.001
Bowie, R. C. K., Fjeldså, J., Hackett, S. J., and Crowe, T. M. (2004). Molecular evolution in space and though time: mtDNA phylogeography of the Olive Sunbird (Nectarinia olivacea/obscura) throughout continental Africa. Mol. Phylogenet. Evol. 33, 56–76. doi: 10.1016/j.ympev.2004.04.013
Bowie, R. C. K., Pasquet, E., McEntee, J. P., Njilima, F., and Fjeldså, J. (2018). The systematics and biogeography of African Tailorbirds (Cisticolidae: artisornis) with comments on the choice of Bayesian branch-length prior when analyzing heterogeneous data. Mol. Phylogenet. Evol. 118, 172–183. doi: 10.1016/j.ympev.2017.08.011
Burgess, N. D., Butynski, T. M., Cordeiro, N. J., Doggart, N. H., Fjeldså, J., Howell, K. M., et al. (2006). The biological importance of the Eastern arc Mountains of Tanzania and Kenya. Biol. Conserv. 134, 209–231.
Burgess, N. D., and Clarke, G. P. (2000). Coastal Forests of Eastern Africa. Gland: IUCN The World Conservation Union.
Burgess, N. D., and Mlingwa, C. O. F. (2000). Evidence for altitudinal migration of forest birds between montane Eastern Arc and lowland forests in East Africa. Ostrich 71, 184–190.
Cadena, C. D., Kozak, K. H., Gómez, J. P., Parra, J. L., McCain, C. M., Bowie, R. C. K., et al. (2012). Latitude, elevational climatic zonation and speciation in New World vertebrates. Proc. R. Soc. B Biol. Sci. 279, 194–201. doi: 10.1098/rspb.2011.0720
Cadena, C. K., and Cespedes, L. N. (2020). “Origin of elevational replacements in a clade of nearly flighless birds: most diversit in tropical mountains accumulates via secondary contact following allopatric speciation,” in Neotropical Speciation, eds V. Rull and A. C. Carnaval (Berlin: Springer), 635–659. doi: 10.1007/978-3-030-31167-4_23
Cai, T., Cibois, A., Alström, P., Moyle, R., Kennedy, J. D., Shao, S., et al. (2019). Near-complete phylogeny and taxonomic revision of the world’s babblers (Aves: passeriformes). Mol. Phylogenet. Evol. 130, 346–356. doi: 10.1016/j.ympev.2018.10.010
Clancey, P. A. (1989). The taxonomy of the Green Barbets (Aves: lybiidae) of the Eastern Afrotropics. Bonn. Zool. Beitr. 40, 11–18.
Clancey, P. A., and Lawson, W. J. (1969). A new race of White-breasted Alethe From Moçambique. Bull. Br. Ornithol. Club 89, 4–6.
Cox, S. C., Prys-Jones, R. P., Habel, J. C., Amakobe, B. A., and Day, J. J. (2014). Niche divergence promotes rapid diversification of East African sky island white-eyes (Aves: zosteropidae). Mol. Ecol. 23, 4103–4118. doi: 10.1111/mec.12840
Cracraft, J. (1983). Species concepts and speciation analysis. Curr. Ornithol. 1, 159–187. doi: 10.1007/978-1-4615-6781-3_6
Crowe, T. M., and Crowe, A. A. (1982). Patterns of distribution, diversity and endemism in afrotropical birds. J. Zool. 198, 417–442. doi: 10.1016/j.ympev.2009.10.027
Davies, O. R. (2014). Taxonomy, phylogeny and biogeography of cisticolas (Cisticola spp.). Ph.D. thesis. Cape Town: Percy FitzPatrick Institute of African Ornithology.
deKlerk, H. M., Crowe, T. M., Fjeldså, J., and Burgess, N. D. (2002). Biogeographical patterns of endemic terrestrial Afrotropical birds. Divers. Distrib. 8, 147–162. doi: 10.1046/j.1472-4642.2002.00142.x
del Hoyo, J., and Collar, N. J. (2016). HBW and BirdLife International Illustrated Checklist of the Birds of the World. Volume 2: passerines. Barcelona: Lynx Edicions.
deMenocal, P. B. (2004). African climate change and faunal evolution during the Pliocene-Pleistocene. Earth Planet. Sci. Lett. 220, 3–24.
Diamond, D. W., and Hamilton, A. C. (1980). The distribution of forest passerine birds and Quaternary climatic change in tropical Africa. J. Zool. 191, 379–402.
Dowsett, R. J. (1986). “Origins of the high-altitude avifaunas of tropical Africa,” in High Altitude Tropical Biogeography, eds F. Vuilleumier and M. Monasterio (Oxford: Oxford University Press), 557–585.
Drummond, A. J., and Rambaut, A. (2007). BEAST: bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7:214. doi: 10.1186/1471-2148-7-214
Fjeldså, J., and Bowie, R. C. K. (2008). New perspectives on Africa’s ancient forest avifauna. Afr. J. Ecol. 46, 235–247. doi: 10.1111/j.1365-2028.2008.00992.x
Fjeldså, J., Bowie, R. C. K., and Kiure, J. (2006). The Forest Batis Batis mixta is two species: description of a new, narrowly distributed Batis species in the Eastern arc biodiversity hotspot. J. Ornithol. 147, 578–590. doi: 10.1007/s10336-006-0082-4
Fjeldså, J., Christidis, L., and Ericson, P. G. P. (2020). The Largest Avian Radiation. The Evolution of Perching Birds, or the Order Passeriformes. Barcelona: Lynx ed.
Fjeldså, J., Dinesen, L., Davies, O. R., Irestedt, M., Krabbe, N. K., Hansen, L. A., et al. (2021). Description of two new cisticola species endemic to the marshes of the Kilombero floodplain of southwestern Tanzania. ibis 163, 1330–1354. doi: 10.1111/ibi.12971
Fjeldså, J., Kiure, J., Doggart, N., Hansen, L. A., and Perkin, A. (2010). Distribution of highland forest birds across a potential dispersal barrier in the Eastern Arc Mountains of Tanzania. Steenstrupia 32, 1–43.
Fjeldså, J., Roy, M. S., and Kiure, J. (2000). A montane subspecies of Sheppardia gunning (East-coast Akalat) from Tanzania. Bull. Br. Ornithol. Club 120, 27–33.
Forestry and Beekeeping Division (2006). “Forest Area Baseline for the Eastern Arc Mountains,” in Conservation and Management of the Eastern Arc Mountain Forests, eds B. P. Mbilinyi, R. E. Malimbwi, D. T. K. Shemwetta, A. Songorwa, E. Zahabu, J. Z. Katani, et al. (Dar es Salaam: Forestry and Beekeeping Division).
Fuchs, J., Bowie, R. C. K., and Fjeldså, J. (2011). Diversification across an altitudinal gradient in the Tiny Greenbul (Phyllastrephus debilis) from the Eastern Arc Mountains of Africa. BMC Evol. Biol. 17:117. doi: 10.1086/1471-2148-11-117
Fuchs, J., and Bowie, R. C. K. Fjeldså, J., and Pasquet, E. (2004). Phylogenetic relationships of the African bush-shrikes and helmet-shrikes (Passeriformes: Malaconotidae). Mol. Phylogenet. Evol. 33, 428–439. doi: 10.1016/j.ympev.2004.06.014
Gill, F., Donsker, D., and Rasmussen, P. (2021). IOC World Bird List (v 11.1). Available Online at: http://www.worldbirdnames.org/ doi: 10.14344/IOC.ML.11.1 (accessed November 24, 2021).
Griffiths, C. J. (1993). “The geological evolution of East Africa,” in Biogeography & ecology of the rain forests of Eastern Africa, eds J. C. Lovett and S. K. Wasser (Cambridge, U.K: Cambridge University Press), 9–21. doi: 10.1017/cbo9780511895692.002
Gwee, C. Y., Garg, K. M., Chattopadhyay, B., Sadanandan, K. P., Prawiradilaga, D. W., Irestedt, M., et al. (2020). Phylogenomics of white-eyes, a ‘great speciator’, reveals Indonesian archipelago s the center of lineage diversity. eLife 9:e62765. doi: 10.7554/eLife.62765
Hall, B. P., and Moreau, R. E. (1970). An Atlas of Speciation in African Passerine Birds. London: British Museum (Natural History).
Harris, R. B., Irwin, K., Jones, M. R., Laurent, S., Barrett, R. D. H., Nachman, M. W., et al. (2020). The population genetics of crypsis in vertebrates: recent insights from mice, hares, and lizards. Heredity 124, 1–14. doi: 10.1038/s41437-019-0257-4
Holt, B., Lessard, J.-P., Borregaard, M. K., Araújo, M., Dimitrov, D., Fabre, P.-H., et al. (2013). A global map of Wallacean biogeographic regions. Science 339, 74–78. doi: 10.1126/science.1228282
Jacobs, B. F., Kingston, J. D., and Jacobs, L. L. (1999). The origin of grass-dominated ecosystems. Ann. Mo. Bot. Gard. 86, 590–643. doi: 10.2307/2666186
Jensen, F. P., Tøttrup, A., and Christensen, K. D. (2005). The avifauna of coastal forests in southeast Tanzania. Scopus 25, 1–22.
Johansson, U. S., Fjeldså, J., Lokugalappatti, L. G. S., and Bowie, R. C. K. (2007). A nuclear DNA phylogeny and proposed taxonomic revision of African Greenbuls (Aves, Passeriformes, Pycnonotidae). Zool. Scr. 36, 417–427.
John, J. R., and Kiwango, H. (2021). Further additions to the avifauna of the Isunkaviola Plateau, Ruaha National Park, south-central Tanzania, emphasize its ornithological importance. Scopus 41, 24–34. doi: 10.4103/0972-4923.201393
Kahindo, C., Bates, J. M., and Bowie, R. C. K. (2017). Population genetic structure of Grauer’s Swamp Warbler Bradypterus graueri, an Albertine Rift endemic. Ibis 159, 415–429.
Katoh, K., and Standley, D. M. (2013). MAFFT Multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. doi: 10.1093/molbev/mst010
Khaliq, I., Fritz, S. A., Prinzinger, R., Pfenninger, M., Böhning-Gaese, K., and Hof, C. (2015). Global variation in thermal physiology of birds and mammals: evidence for phylogenetic niche conservatism only in the tropics. J. Biogeogr. 42, 2187–2196. doi: 10.1111/jbi.12573
Kimball, R. T., Braun, E. L., Barker, F. K., Bowie, R. C. K., Braun, M. J., Chojnowski, J. L., et al. (2009). A well-tested set of primers to amplify regions spread across the avian genome. Mol. Phylogenet. Evol. 50, 654–660. doi: 10.1016/j.ympev.2008.11.018
Lawson, L. P. (2013). Diversification in a biodiversity hot spot: landscape correlates of phylogeographic patterns in the African spotted reed frog. Mol. Ecol. 22, 1947–1960. doi: 10.1111/mec.12229
Lerner, H. R. L., Meyer, M., James, H. F., Hofreiter, M., and Fleischer, R. C. (2011). Multilocus resolution of phylogeny and timescale in the extant adaptive radiation of Hawaiian Honeycreepers. Curr. Biol. 21, 1838–1844. doi: 10.1016/j.cub.2011.09.039
Linck, E. B., Freeman, B. G., Cadena, C. D., and Ghalambor, C. K. (2021). Evolutionary conservatism will limit responses to climate change in the tropics. Biol. Lett. 17:20210363. doi: 10.1098/rsbl.2021.0363
Linder, P., de Klerk, H., Born, J., Burgess, N., Fjeldså, J., and Rahbek, C. (2012). The partitioning of Africa: statistically defined biochorological zones in sub-Saharan Africa. J. Biogeogr. 39, 1189–1205.
Lokugalappatti, L. G. S. (2011). Climatic perturbations and speciation of southern and eastern African greenbuls (Passeriformes, Pycnonotidae). Ph.D. thesis. South Africa: Stellenbosch University.
Lovett, J. C. (1993). “Climatic history and forest distribution in eastern Africa,” in Biogeography & ecology of the rain forests of Eastern Africa, eds J. C. Lovett and S. K. Wasser (Cambridge U.K: Cambridge University Press), 23–29. doi: 10.1017/cbo9780511895692.003
Lovett, J. C., and Wasser, S. K. (1993). Biogeography & ecology of the rain forests of Eastern Africa. Cambridge U.K: Cambridge University Press.
Lyons, R. P., Scholz, C. A., Cohen, A. S., King, J. W., Brown, E. T., Ivory, S. J., et al. (2015). Continuous 1.3 million-year record of East African hydroclimate, and implications for patterns of evolution and biodiversity. Proc. Natl. Acad. Sci. U. S. A. 112, 15568–15573. doi: 10.1073/pnas.1512864112
Marchant, R., Mumbi, C., Behera, S., and Yamagata, T. (2007). The Indian Ocean dipole – the unsung driver of climatic variability in East Africa. Afr. J. Ecol. 45, 4–16.
Martins, F. C., Cox, S. C., Irestedt, M., Prys-Jones, R. P., and Day, J. J. (2020). A comprehensive molecular phylogeny of Afrotropical white-eyes (Aves: zosteropidae) highlights prior underestimation of mainland diversity and complex colonization history. Mol. Phylogenet. Evol. 149:106843. doi: 10.1016/j.ympev.2020.106843
Miller, M. A., Pfeiffer, W., and Schwartz, T. (2010). “Creating the CIPRES science gateway for inference of large phylogenetic trees,” in 2010 Gateway Computing Environments Workshop (GCE). (New Orleans, LA, USA: IEEE), doi: 10.1109/GCE.2010.5676129
Mittermeier, R. A., Robles-Gill, P., Hoffmann, M., Pilgrim, J. D., Brooks, T. B., Mittermeier, C. G., et al. (2004). Hotspots revisited. Earth’s Biologically Richest and most Endangered Ecoregions. Mexico City: CEMEX.
Mulwa, R. K., Neuschulz, E. L., Böhning-Gaese, K., and Schleuning, M. (2012). Seasonal fluctuations of resources and avian feeding guilds across forest-farmland boundaries in tropical Africa. Oikos 122, 524–532.
Mumbi, C. T., Marchant, R., Hooghiemstra, H., and Woeller, M. J. (2008). Late Quaternary vegetation reconstruction from the Eastern Arc Mountains, Tanzania. Quat. Res. 69, 326–341.
Nguembock, B., Cibois, A., Bowie, R. C. K., Cruaud, C., and Pasquet, E. (2009). Phylogeny and biogeography of the genus Illadopsis (Passeriformes: timaliidae) reveal the complexity of diversification on some African taxa. J. Avian Biol. 40, 113–125. doi: 10.1111/j.1600-048x.2009.04410.x
Nguembock, B., Fjeldså, J., Couloux, A., Cruaud, C., and Pasquet, E. (2008). Polyphyly of the genus Apalis and a new generic name for the species pulchra and ruwenzorii. Ibis 150, 756–765.
Nicholson, S. E. (2000). The nature of rainfall variation over Africa on time scales of decades to millennia. Glob. Planet. Change 26, 137–153. doi: 10.1016/s0921-8181(00)00040-0
Päckert, M., Martens, J., Sun, Y.-H., Severinghaus, I. I., Nazarenko, A. A., Ting, J., et al. (2012). Horizontal and elevational phylogenographic patterns of Himalayan and Southeast Asian forest passerines (Aves: passeriformes). J. Biogeogr. 39, 556–573. doi: 10.1111/j.1365-2699.2011.02606.x
Pearson, D. J., and Turner, D. A. (2017). A taxonomic review of the genus Zosterops in East Africa, with revised list of species occurring in Kenya, Uganda and Tanzania. Scopus 37, 1–13.
Portik, D. M., Bell, R. C., Blackburn, D. C., Bauer, A. M., Barratt, C. D., Branch, W. R., et al. (2019). Sexual dimorphism drives diversification within a major radiation of African amphibians. Syst. Biol. 68, 859–875. doi: 10.1093/sysbio/syz023
Prell, W. I., Hutson, W. H., Williams, D. F., Bé, A. W. H., Geitzenauer, K., and Molfino, B. (1980). Surface circulation of the Indian Ocean during the Last Glacial Maximum, approximately 18.000 yr BP. Quat. Res. 14, 309–336.
Rovero, F., Menegon, M., Fjeldså, J., Collett, L., Doggart, N., Leonard, C., et al. (2014). Targetted vertebrate surveys enhance the faunal importance and improve explanatory models within the Eastern Arc Mountains of Kenya and Tanzania. Divers. Distrib. 20, 1438–1449.
Ryan, P. G. (2006). “Family Cisticolidae (Cisticolas and allies),” in Handbook of the Birds of the World, eds J. del Hoyo, A. Elliott, and D. Christie (Barcelona: Lynx Ed), 378–491.
Sarmiento, G. (1986). “Ecological features of climate in high tropical mountains,” in High Altitude Tropical Biogeography, eds F. Vuilleumier and M. Monasterio (Oxford: Oxford University Press), 11–45.
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Strömberg, C. A. E. (2011). Evolution of grasses and grassland ecosystems. Ann. Rev. Earth Plant. Sci. 39, 517–544.
Stuart, S. N. (1981). An explanation for the disjunct distributions of Modulatrix orostruthus and Apalis (or Orthotomus) moreaui. Scopus 5, 1–4. doi: 10.1007/978-3-319-23534-9_1
Swofford, D. L. (2002). PAUP*10b: phylogenetic Analysis Using Parsimony (*And other Methods). Sutherland, Massachusetts: Sinauer Ass.
Trauth, M. H., Maslin, M. A., Deino, A., and Strecker, M. R. (2005). Late Cenozoic moisture history of East Africa. Science 309, 2051–2053. doi: 10.1126/science.1112964
Voelker, G., and Light, J. E. (2011). Paleoclimate events, dispersal and migratory losses along the Afro-European axis as drivers of biogeographic distribution in Sylvia warblers. BMC Evol. Biol. 11:163. doi: 10.1186/1471-2148-11-163
Voelker, G., Melo, M., and Bowie, R. C. K. (2009). A Gulf of Guinea endemic is a member of a Mediterranean-centred bird genus. Ibis 151, 580–583. doi: 10.1111/j.1474-919x.2009.00934.x
Voelker, G., Outlaw, R. K., and Bowie, R. C. K. (2010). Pliocene forest dynamics as a primary driver of African bird speciation. Glob. Ecol. Biogeogr. 19, 111–121.
Vrba, E. S., Denton, G. H., Partridge, T. C., and Burckle, L. H. (1995). Paleoclimate and Evolution, with Emphasis on Human Origins. New Haven: Yale University Press.
White, F. (1981). The history of the afromontane archipelago and the scientific need for its conservation. Afr. J. Ecol. 19, 33–54. doi: 10.1111/j.1365-2028.1981.tb00651.x
Winger, B. M., Auteri, G. G., Pegan, T. M., and Weeks, B. C. (2018). A long winter for the Red Queen: rethinking the evolution of seasonal migration. Biol. Rev. 94, 737–753. doi: 10.1111/brv.12476
Keywords: hotspot, birds, phylogeography, dispersal, corridors
Citation: Fjeldså J and Bowie RCK (2021) Evolutionary and Ecological Explanations for the Elevational Flexibility of Several East African Bird Species Complexes. Front. Ecol. Evol. 9:768062. doi: 10.3389/fevo.2021.768062
Received: 31 August 2021; Accepted: 15 November 2021;
Published: 08 December 2021.
Edited by:
Mauro Fois, University of Cagliari, ItalyReviewed by:
Anna Mária Csergõ, Hungarian University of Agriculture and Life Sciences, HungaryXuelong Jiang, Kunming Institute of Zoology, China
Ara Monadjem, University of Eswatini, Eswatini
Copyright © 2021 Fjeldså and Bowie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jon Fjeldså, JFjeldsaa@snm.ku.dk
†These authors have contributed equally to this work
 Jon Fjeldså
Jon Fjeldså Rauri C. K. Bowie
Rauri C. K. Bowie